Interstitial sites in close packing
Even in the compact packing of the spheres, there is left some empty space between spheres. This empty space in the crystal lattice is known as site or void or hole. The types of voids are given as follows,
(1) Trigonal void : This site is formed when three spheres lie at the vertices of the equilateral triangle. Size of the trigonal site can be given by the following relation,
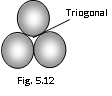
r = 0.155R
r = Radius of the spherical trigonal void
R = Radius of closely packed spheres
(2) Tetrahedral void : A tetrahedral void is developed when triangular voids (made by three spheres in one layer touching each other) have contact with one sphere either in the upper layer or in the lower layer.

Number of the tetrahedral voids is double the number of spheres in the crystal structure.
r/R = 0.225
here, r is the radius of the tetrahedral void or the atom occupying tetrahedral void.
R is the radius of the spheres forming the tetrahedral void.
(3) Octahedral void : This type of void is surrounded by six closely packed spheres, that is it is formed by six spheres.
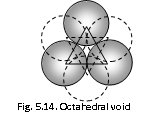
The Number of the octahedral voids is equal to the number of the spheres.
r/R = 0.414
(4) Cubic void : This type of void is formed between 8 closely packed spheres which occupy all the eight cubes corners
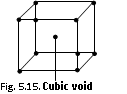
r/R = 0.732
The decreasing order of size of the specified voids is,
Cubic > Octahedral > Tetrahedral > Trigonal
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Interstitial sites in close packing questions? Interstitial sites in close packing topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Interstitial sites in close packing related problems. We provide step by step Interstitial sites in close packing question's answers with 100% plagiarism free content. We prepare quality content and notes for Interstitial sites in close packing topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours