Ideal gas equation
(1) The simple gas laws relating gas volume to temperature pressure, and amount of gas, respectively and are stated as follows :
Boyle's law : 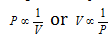 (n and T constant)
(n and T constant)
Charle's law : 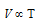 (n and P constant)
(n and P constant)
Avogadro's law : 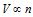 (T and P constant)
(T and P constant)
If all the above law's combines, then it becoms
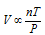
or 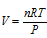 (R = Ideal gas constant)
(R = Ideal gas constant)
or PV = nRT
This is called as ideal gas equation. R is called as the ideal gas constant. This equation is obeyed by the isothermal and adiabatic processes.
(2) The Nature and the values of R: From ideal gas equation can be written as follows,

R is expressed in unit of work or energy mol-1 K-1.
Since different values of R are summarized as follows:
R = 0.0821 L atm mol-1 K-1

(3) Gas constant, R for a single molecule is called Boltzmann constant (k)
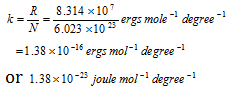
(4) The calculation of mass, molecular weight and density of the gas by gas equation
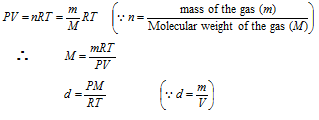
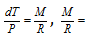
( Here M and R are constant for the particular gas)
Therefore, 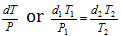 = Constant
= Constant
(For the two or more different temperature and pressure)
(5) The Gas densities differ from those of the solids and liquids as,
(i) The Gas densities are usually stated in g/L instead of g/cm3.
(ii) The Gas densities are strongly dependent on the pressure and temperature as, 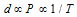
The densities of liquids and solids depend somewhat on temperature, but they are far less dependent on the pressure.
(iii) The density of a gas is directly proportional to its molar mass. There is simple relationship existing between the density and the molar mass for liquid and solids.
(iv) Density of a gas at STP = molar mass/22.4
d(N2) at STP = 28/22.4 = 1.25 g L-1,
d(O2) at STP = 32/22.4 = 1.43 g L-1
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Ideal gas equation questions? Ideal gas equation topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Ideal gas equation related problems. We provide step by step Ideal gas equation question's answers with 100% plagiarism free content. We prepare quality content and notes for Ideal gas equation topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours