Hydrogen Spectrum
(1) The hydrogen spectrum is an example of the line emission spectrum or an atomic emission spectrum.
(2) When the electric discharge is passed through the hydrogen gas at low pressure, a bluish light is emitted from it.
(3) This light shows the discontinuous line spectrum of several isolated sharp lines through the prism.
(4) All these lines of H-spectrum contain Lyman, Balmer, Paschen, Barckett, Pfund and Humphrey series in order. These spectral series were named by the name of scientist discovered them.
(5) To calculate the wavelength of various H-lines Ritz introduced the following expression,
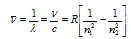
R is the universal constant hear generally known as Rydberg's constant its value is 109, 678 cm-1.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Hydrogen Spectrum questions? Hydrogen Spectrum topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Hydrogen Spectrum related problems. We provide step by step Hydrogen Spectrum question's answers with 100% plagiarism free content. We prepare quality content and notes for Hydrogen Spectrum topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours