Hydrogen peroxide
Hydrogen peroxide (H2O2) was discovered by French chemist Thenard.
(1) Preparation : It is prepared by
(i) Laboratory method : In laboratory, H2O2 can be prepared by Merck's procedure. It is prepared by adding calculated amounts of sodium peroxide to ice cold dilute (20%) solution of H2SO4.

(ii) By the action of the sulphuric acid or phosphoric acid on hydrated barium peroxide BaO2.8H2O
(a) 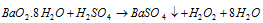
It must be noted that anhydrous barium peroxide does not react readily with sulphuric acid (because a coating of insoluble barium sulphate is formed on its surface which stops further action of the acid). Therefore, hydrated barium peroxide, BaO2.8H2O should be taken in use.
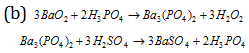
Phosphoric acid is preferred to H2SO4 because soluble impurities like barium persulphate (from BaO2.8H2O + H2SO4) tends to decompose H2O2 while H3PO4 acts as preservative (negative catalyst) for H2O2.
(iii) Industrial method : On a commercial scale, H2O2 can be prepared by the electrolysis of 50% H2SO4 solution. In a cell, peroxy disulphuric acid is formed at the anode.
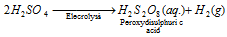
This is drawn off from the cell and hydrolysed with water to give H2O2.
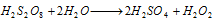 The resultant solution is then distilled under reduced pressure when H2O2 gets distilled while H2SO4 with high boiling point, remains undistilled.
The resultant solution is then distilled under reduced pressure when H2O2 gets distilled while H2SO4 with high boiling point, remains undistilled.
(iv) By redox process : Industrially H2O2 is prepared by the auto-oxidation of 2-alkylanthraquinols. The process includes a cycle of reactions. The average reaction is the catalytic union of H2 and O2 to give H2O2.
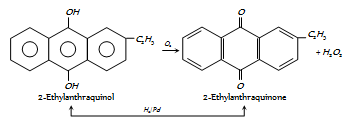
The H2O2 formed (about 1%) is extracted with water and concentrated.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Hydrogen peroxide, Preparation of Hydrogen peroxide questions? Hydrogen peroxide, Preparation of Hydrogen peroxide topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Hydrogen peroxide, Preparation of Hydrogen peroxide related problems. We provide step by step Hydrogen peroxide, Preparation of Hydrogen peroxide question's answers with 100% plagiarism free content. We prepare quality content and notes for Hydrogen peroxide, Preparation of Hydrogen peroxide topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours