Higher fatty acids
Palmitic, stearic and oleic acids are found in natural fats and oils as glyceryl esters.
They have derived their names from the natural source from which they are prepared by hydrolysis with alkali.
Table :
|
Name of acids
|
Source
|
Molecular formula
|
|
Palmitic acid
|
Palm oil
|
CH3(CH2)14COOH
|
|
Stearic acid
|
Stear (meaning tallow)
|
CH3(CH2)16COOH
|
|
Oleic acid
|
Olive oil.
|
CH3(CH2)7CH = CH(CH2)7COOH
|
Palmitic and stearic acids are waxy colourless solids with melting points 64°C and 72°C, respectively. They are insoluble in water but soluble in ethanol and ether. They find use in the manufacture of soaps and candles. Soaps contain sodium or potassium salts of these higher fatty acids.
Oleic acid has low melting point, i.e., 16°C. It is insoluble in water but soluble in alcohol and ether. Besides the reactions of acids, it also gives reactions of alkenes. Two aldehydes are formed on ozonolysis.
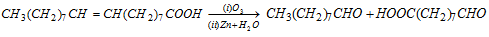
It is used for making soaps, lubricants and detergents.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Higher fatty acids questions? Higher fatty acids topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Higher fatty acids related problems. We provide step by step Higher fatty acids question's answers with 100% plagiarism free content. We prepare quality content and notes for Higher fatty acids topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours