Hard and Soft water
Water which produces lather with soap solution readily is called soft water. For example rain water distilled water, and water which is not mineralized water.
Water which does not produce lather with soap solution readily is called hard water. For example sea water, river water, well water and tap water.
(i) Cause of hardness of water : The hardness of water is due to the presence of bicarbonates, chlorides and sulphates of calcium and magnesium.
Hard water does not produce lather because the cations (Ca2+ and Mg2+) present in hard water react with soap to form insoluble precipitates,
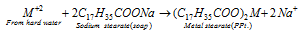 Where M = Ca or Mg
Where M = Ca or Mg
Therefore, no lather is produced until all the calcium and magnesium ions are precipitated. This generally results into the wastage of lot of soap.
(ii) Type of hardness of water : The hardness of water is of two types,
(a) Temporary hardness : This is due to the presence of bicarbonates of calcium and magnesium. It is known as carbonate hardness also.
(b) Permanent hardness : This is because of the presence of chlorides and sulphates of calcium and magnesium. It is also known as non-carbonate hardness.
(iii) Softening of water : The process of the removal of hardness from water is called softening of water.
(a) Removal of temporary hardness : It can be removed by the following methods,
- By boiling : While boiling, the bicarbonates of Ca and Mg decompose into insoluble carbonates and give CO2. The insoluble carbonates can be removed by filtration.
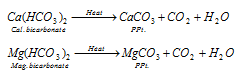
- Clark's method : This process is used on a commercial scale. In this method, calculated amount of lime is added to temporary hard water.
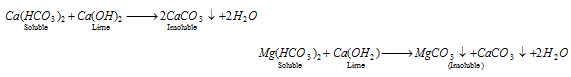
(b) Removal of the permanent hardness : The permanent hardness can be removed by following methods,
- By washing soda method : In this method, water is treated with a calculated amount of washing soda (Na2CO3) which converts the chlorides and sulphates of Ca and Mg into their respective carbonates which get precipitated.
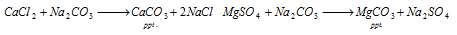
- Permutit method : This is a modern method employed for the softening of hard water. hydrated sodium aluminium silicate (Na2Al2Si2O8.xH2O) is called permutit. These compound salts are also called as zeolites.
The permutit as loosely packed in a big tank over a layer of coarse sand. The hard water is introduced into the tank from the top. The water reaches bottom of the tank and then slowly increases its level through the permutit layer in the tank. The cations their in hard water are exchanged for sodium ions. Thus this method is also called ion exchange method.
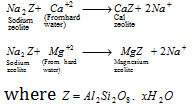
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Hard and Soft Water questions? Hard and Soft Water topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Hard and Soft Water related problems. We provide step by step Hard and Soft Water question's answers with 100% plagiarism free content. We prepare quality content and notes for Hard and Soft Water topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours