Gravimetric analysis: In the gravimetric analysis we relate the weights of the two substances or a weight of a substance with the volume of a gas or the volumes of two or more gases.
The problems Involving Mass-Mass Relationship
Proceed for solving such type of problems according to the following instructions,
(i) Write the balanced equation to depict the chemical change.
(ii) Write the number of moles below the formula of reactants and products. Also write down the relative weights of the reactants and products (calculated from respective molecular formula), below the respective formula are given.
(iii) Apply unitary method to calculate the unknown factor (s).
The problems involving the Mass-Volume Relationship
For solving the problems involving mass-volume relationship, proceed according to the instructions given below,
(i) Write the relevant balanced chemical equations (s).
(ii) Write the weights of the various solid reactants and products.
(iii) Gases are generally expressed in terms of volumes. In the case the volume of the gas is measured at the room temperature and pressure (or under the conditions other than N.T.P.), convert it into the N.T.P. by applying gas equation.
(iv) Volume of the gas at any temperature and pressure can be converted into its weight and vice-versa with the help of relation, by 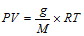 where g is weight of gas, M is mole. wt. of gas, R is gas constant.
where g is weight of gas, M is mole. wt. of gas, R is gas constant.
Calculate the unknown factor by the unitary method.
The problems Based on Volume-Volume Relationship
Such type of problems can be solved according to the chemical equation as,
(i) Write the relevant balanced chemical equation.
(ii) Write the volume of reactants and products below the formula to each of the reactant and product with help of the fact that 1 gm molecule of every gaseous substance occupies the volume of 22.4 litres at N.T.P.
(iii) In case volume of gas is measured under particular (or in the room) temperature, convert it to volume at NTP by using the ideal gas equation.
By taking the help of the Avogadro's hypothesis "Equal volume of the different gases under the similar conditions of temperature and pressure contain same number of molecules".
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Gravimetric analysis questions? Gravimetric analysis topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Gravimetric analysis related problems. We provide step by step Gravimetric analysis question's answers with 100% plagiarism free content. We prepare quality content and notes for Gravimetric analysis topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours