Fuel cells
These are Voltaic cells in which the reactants are continuously supplied to the electrodes. These cells are designed to convert the energy from the combustion of fuels like H2, CO, CH4, etc. directly into electrical energy. The general example is hydrogen-oxygen fuel cell described as follows,
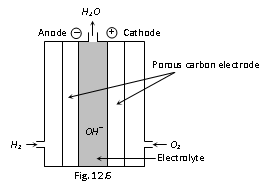
In this cell, hydrogen and oxygen are bubbled through a porous carbon electrode into the concentrated aqueous sodium hydroxide or the potassium hydroxide. The hydrogen (which is fuel) is fed into the anode compartment where it is oxidized. The oxygen is fed inside the cathode compartment where it is reduced. Diffusion rates of the gases into the cell are carefully regulated to get maximum efficiency. Net reaction is the same as burning of hydrogen and oxygen to form water. The reactions can be given as follows
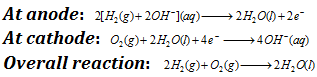
Each electrode is made of porous compressed carbon containing a small amount of catalyst Pt, Ag, CoO. This cell runs continuously as long as the reactants are fed. The Fuel cells convert energy of the fuel
directly into the electricity EMF of fuel cell is 1.23 V. The cell has been taken in use for electric power in the Apollo space programme. The significant advantages of fuel cells are
(1) High efficiency : The fuel cells convert the energy of a fuel directly into electricity and thus, they are much more efficient than the conventional methods of generating electricity on a large scale by burning hydrogen, carbon fuels. Though we expect 100 % efficiency in fuel cells, so far 60 - 70% efficiency has been attained. A conventional method of the production of electrical energy includes combustion of a fuel to liberate heat which is then used to produce electricity. Efficiency of these methods is only about 40%.
(2) Continuous source of energy : There is no electrode material to be replaced as in ordinary battery. The fuel is fed continuously to produce the power. For this reason, H2-O2 fuel cells have been used in the space crafts.
(3) Pollution free working: There are no objectionable byproducts and, thus, they do not cause the pollution problems. As fuel cells are efficient and free from the pollution, attempts are being made to obtain better commercially practical fuel cells.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Fuel cells questions? Fuel cells topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Fuel cells related problems. We provide step by step Fuel cells question's answers with 100% plagiarism free content. We prepare quality content and notes for Fuel cells topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours