Free energy and Free energy change
The Gibb's free energy (denoted by G) is a state function and is a measure of maximum work done or useful work done from a reversible reaction at constant temperature and pressure.
(1) Characteristics of free energy
(i) The systems free energy is the enthalpy of the system minus the product of absolute temperature and entropy which means G=H-TS
(ii) Like other state functions E, H and S, it is also expressed as ΔG. Also ΔG = ΔH - TΔS where ΔS is entropy change for system only. This is the Gibb's Helmholtz equation.
(iii) At equilibrium ΔG = 0
(iv) For the spontaneous process decrease in free energy is noticed that is ΔG = -ve.
(v) At absolute zero, TΔS is zero. Therefore if ΔG is - ve, ΔH should be - ve or only exothermic reactions proceed spontaneously at absolute zero.
(vi) 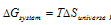 , where ΔH = 0
, where ΔH = 0
(vii) The standard free energy change, 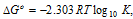
where K is equilibrium constant.
(a) Thus if K>1 then ΔG0 = -ve thus reactions with equilibrium constant K>1 are thermodynamically spontaneous.
(b) If K<1, then ΔG0 = +ve and thus reactions with equilibrium constant K<1 are thermodynamically spontaneous in reverse direction.
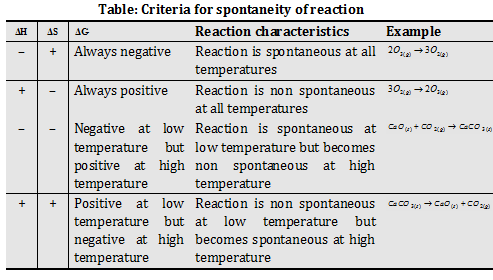
(2) Criteria for spontaneity of reaction : For a spontaneous change ΔG = -ve and therefore use of
ΔG = ΔH - TΔS provides the following conditions for a change to be spontaneous.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Free energy and Free energy change questions? Free energy and Free energy change topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Free energy and Free energy change related problems. We provide step by step Free energy and Free energy change question's answers with 100% plagiarism free content. We prepare quality content and notes for Free energy and Free energy change topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours