Formic Acid or Methanoic acid (HCOOH)
Formic acid is the first member of monocarboxylic acids series. It occurs in the sting of bees, wasps, red ants, stinging nettles and fruits. In traces it is present in perspiration, urine, blood and in caterpillars.
(1) Methods of preparation
(i) Oxidation of methyl alcohol or formaldehyde
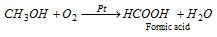
(ii) Hydrolysis of hydrocyanic acid : Formic acid is formed by the hydrolysis of HCN with acids or alkalies.
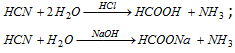
(iii) Laboratory preparation
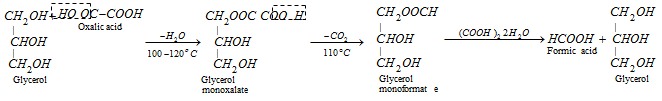
The following procedure is applied for obtaining anhydrous formic acid.
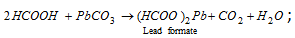 ;
;
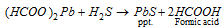
(iv) Industrial preparation : Formic acid is prepared on industrial scale by heating sodium hydroxide with carbon monoxide at 210°C under a pressure of about 10 atmospheres.
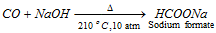
Sodium formate thus formed is distilled with sodium hydrogen sulphate, when anhydrous formic acid distils over.
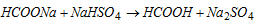
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with formic acid or methanoic acid questions? formic acid or methanoic acid topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their formic acid or methanoic acid related problems. We provide step by step formic acid or methanoic acid question's answers with 100% plagiarism free content. We prepare quality content and notes for formic acid or methanoic acid topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours