|
Crystal system
|
Space lattice
|
Examples
|
|
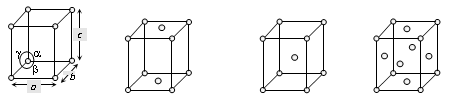
Cubic
A=b=c,
α = β = ϒ= 90o
|
Simple: Lattice points at the eight corners of the unit cells.
|
Body centered: Points at the eight corners and at the body centred.
|
Face centered: Points at the eight corners and at the six face centres.
|
Pb, Hg, Ag Au, Cu, ZnS, diamond, KCl, CsCl, NaCl, Cu2O, CaF2 and alums. etc.
|
|
Tetragonal
a = b ≠ c,
α =β = ϒ = 90o
|
Simple : Points at the eight corners of the unit cell.

|
Body centered : Points at the eight corners and at the body centre
|
SnO2, TiO2
ZnO2, NiSO4
ZrSiO4.PbW O4, white Sn etc.
|
|
Orthorhombic (Rhombic)
a ≠b ≠ c,
α =β = ϒ = 90o
|
Simple: Points at the eight corners of the unit cell.
|
End centered: Also called side centered or base centered. Points at the eight corners and at two face centres opposite to each other.
|
Body centered : Points at the eight corners and at the body centre
|
Face centered: Points at the eight corners and at the six face centres.
|
KNO3, K2SO4
PbCO3, BaSO4, rhombic sulphur, MgSO4.7H2O etc.
|
|
Rhombohedral
or Trigonal
a = b = c,
α =β = ϒ ≠ 90o
|
Simple : Points at the eight corners of the unit cell
|
NaNO3, CaSO4, calcite, quartz, As, Sb, Bi etc.
|
|
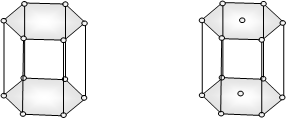
Hexagonal
a = b ≠ c,
α =β = 90o
ϒ = 120o
|
Simple: Points at the twelve corners of the unit cell out lined by thick line.
|
or Points at the twelve corners of the hexagonal prism and at the centres of the two hexagonal faces.
|
ZnO, PbS, CdS, HgS, graphite, ice, Mg, Zn, Cd etc.
|
|
Monoclinic
a ≠ b ≠ c,
α = ϒ= 90o
β ≠ 90o
|
Simple : Points at the eight corners of the unit cell

|
End centered : Point at the eight corners and at two face centres opposite to the each other.
|
Na2SO4.10H2O
Na2B4O7.10H2O
CaSO4.2H2O monoclinic sulphur etc.
|
|
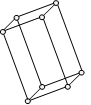
Triclinic
a ≠ b ≠ c,
α ≠ β ≠ ϒ= 90o
|
Simple : Points at the eight corners of the unit cell.
|
CaSO4.5H2O K2Cr2O7, H3BO3
etc.
|
|
|
|
|
| |
|
|
|
|
|
|
|
|