Formation of coloured ions:
Many compounds of the transition elements are coloured that are available in the solid state and /or also in the solution phase. Because of the presence of unpaired electrons in their d-orbitals the compounds of transition metals are coloured.
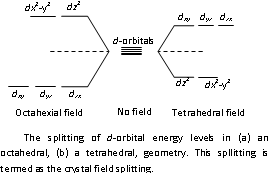
Description: within an isolated ion or atom of transition elements these all five d-orbitals are of the similar energy which are said to be regenerate. In the influence of the electron rich molecules and the combining anion there are five d-orbitals split within two (or sometimes more than two) levels of the different energies. There are the difference in these two energy levels that depends upon the nature of the combining ions here corresponds to the energy associated along with the radiations in the visible region, (λ = 380 - 760 nm). Classically splitting for tetrahedral and octahedral geometries is display in the above figure.
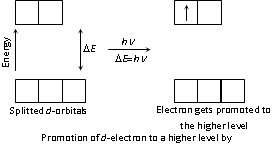
It has one or more unpaired electrons in the transition metal in elements form or in the ionic form. When the visible light falls on the sample at that time the lower energy electrons get promoted to the higher energy level while the absorption of light of the features wavelength or colour. It depends upon the energy difference of the two levels rather it is lower or higher. The other light gets transmitted in a colour complementary to absorbed colour. Therefore, the compound or the solution appears to be of complementary colour. Here is the short example, Cu(H2O)62+ ions absorb red radiation and it looks blue-green the blue-green is the complementary colour to red. The hydrated Co2+ ions absorb radiation in blue-green region, and so, appear red in sunlight.
|
Colour of the
|
Colour of the
|
|
Absorbed light
|
Transmitted light
|
Absorbed light
|
Transmitted light
|
|
IR
|
White
|
Blue-green
|
Red
|
|
Red
|
Blue-green
|
Blue
|
Orange
|
|
Orange
|
Blue
|
Indigo
|
Yellow
|
|
Yellow
|
Indigo
|
Violet
|
Yellow-green
|
|
Yellow-green
|
Violet
|
UV
|
White
|
|
Green
|
Purple
|
|
|
Relationship in the colour of the absorbed radiation and that of transmitted light is providing in above table.
Thus, the colour of the substance will be the colour of the transmitted radiation if radiations of all the wavelengths (or colours) accept one is absorbed. The example for that is as follows, if a substance absorbs all colours except green so it would display green to the eyes.
The transition metal ions which will completely filled by the d-orbitals are basically colourless, as there are no vacant d-orbitals to allow the promotion of the electrons. However, Zn2+ (3d10), Cd2 + (4d10) and Hg2+(5d10) Sc 3+, Ti4+, Cu+ ions and Zn, Cd, Hg are also colourless and diamagnetic. The empty transition metal ions d-orbitals are also colourless, Therefore, Sc3+ and Ti4+.ions are colourless, unless a coloured anion is present in the compound.
|
Ion
|
Outer configuration
|
Number of unpaired electrons
|
Colour of the ion
|
|
Sc3+
|
3d0
|
0
|
Colourless
|
|
Ti3+
|
3d1
|
1
|
Purple
|
|
Ti4+
|
3d0
|
0
|
Colourless
|
|
V3+
|
3d2
|
2
|
Green
|
|
Cr3+
|
3d3
|
3
|
Violet
|
|
Mn2+
|
3d5
|
5
|
Light pink
|
|
Mn3+
|
3d4
|
4
|
Violet
|
|
Fe2+
|
3d6
|
4
|
Green
|
|
Fe3+
|
3d5
|
5
|
Yellow
|
|
Co3+
|
3d7
|
3
|
Pink
|
|
Ni2+
|
3d8
|
2
|
Green
|
|
Cu2+
|
3d9
|
1
|
Blue
|
|
Cu+
|
3d10
|
0
|
Colourless
|
|
Zn2+
|
3d10
|
0
|
Colourless
|
Outer- electronic and Colours the configurations of a few significant ions of the first transition series elements are given above table.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Formation of Coloured ions questions? Formation of Coloured ions topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Formation of Coloured ions related problems. We provide step by step Formation of Coloured ions question's answers with 100% plagiarism free content. We prepare quality content and notes for Formation of Coloured ions topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours