Flocculation or Precipitation or Coagulation
The phenomenon of the precipitation of a colloidal solution by the addition of the excess of an electrolyte is called coagulation or flocculation.
Coagulation of the lyophobic solutions can be carried out by the following methods.
(1) By electrophoresis : In electrophoresis the colloidal particles move towards oppositely charged electrode. These when come in contact with the electrode for long these are discharged and precipitated.
(2) By mixing two oppositely charged solutions : When oppositely charged sols are mixed in almost equal proportions, their charges are neutralized. Both the solutions can be partially or completely precipitated as the mixing of ferric hydroxide (+ve sol) and arsenious sulphide (-ve sol) bring them in precipitated form. This kind of coagulation is called as mutual coagulation or the meteral coagulation.
(3) By boiling : When a sol is boiled, the adsorbed layer is disturbed because of increased collisions with the molecules of dispersion medium. This decreases the charge on the particles and ultimately they settle down to form a precipitate.
(4) By persistent dialysis : On the prolonged dialysis, traces of the electrolyte there in the solution are removed almost completely and the colloids turn out to be unstable.
(5) By addition of electrolytes: The particles of the dispersed phase that is colloids bear some charge. At the time when an electrolyte is added to the solution, the colloidal particles take up ions carrying opposite charge from the electrolyte. As a result, their charge gets neutralized and this causes the uncharged, particles to come near and to get coagulated or precipitated. For instance, if solution is added to sol the ions are attracted by the negatively charged sol particles and their charge gets neutralized. This lead to the coagulation.
(6) Hardy schulze rule: The coagulation capacity of different electrolytes is different. It is dependent upon the valency of the active ion are called as the flocculating ion, which is ion carrying the charge opposite to charge on the colloidal particles. In accordance to Hardy Schulze rule states that, greater the valency of the active ion or flocculating ion, greater is its coagulating power hence, Hardy Schulze law state that:
(i) Ions carrying the charge opposite to that of sol particles are effective in causing coagulation of the sol.
(ii) Coagulating power of the electrolyte is directly proportional to valency of the active ions (ions creating coagulation).
For example to coagulate negative sol of As2S3, the coagulation power of different cations has been found to decrease in the order as,
Al3+ > Mg2+ > Na+
Similarly, to coagulate a positive sol such as Fe(OH)3, the coagulating power of different anions has been found to decrease in the order : [Fe(CN)6]4- > PO43- > SO42- > Cl-
(7) Coagulation or flocculation value
Minimum concentration of electrolyte which is needed to cause the coagulation or the flocculation of a solution is called as flocculation value.
or
Number of the millimoles of an electrolyte desired to bring about the coagulation of one litre of a colloidal solution is called its flocculation value.
Coagulation value or flocculating value 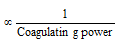
(8) Coagulation of lyophilic sols
(i) There are majorly two factors which are responsible for the stability of lyophilic sols.
(ii) These factors can be the charge and solvation of the colloidal particles.
(iii) When the two factors are removed, the lyophilic solution can be coagulated.
(iv) This can be done
(i) by addition of the electrolyte
(ii) and by addition of the suitable solvent.
(v) When solvent such as alcohol and acetone are added to hydrophilic sols the dehydration of dispersed phase occurs. Under such type of condition a small quantity of electrolyte can bring about coagulation.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Flocculation or Precipitation or Coagulation questions? Flocculation or Precipitation or Coagulation topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Flocculation or Precipitation or Coagulation related problems. We provide step by step Flocculation or Precipitation or Coagulation question's answers with 100% plagiarism free content. We prepare quality content and notes for Flocculation or Precipitation or Coagulation topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours