Factors affecting the extent of adsorption : The following are factors which affect the adsorption process,
(1) Nature of adsorbate (gas) and the adsorbent (solid)
(i) In general, simply liquefiable gases for example CO2, NH3, Cl2 and SO2 etc. are adsorbed to a greater extent than the elemental gases for example H2, O2, N2, He etc. (while chemisorption is specific in nature.)
(ii) Porous and finely powdered solid for example charcoal, fullers earth, adsorb more as compared to the hard non-porous materials. Because of this property the powdered charcoal is taken in use in the gas masks.
(2) The surface area of solid adsorbent
(i) The extent of adsorption depends directly upon the surface area of the adsorbent, which means larger the surface area of the adsorbent, greater will be the extent of adsorption.
(ii) Surface area of a powdered solid adsorbent depends upon its particle size. Smaller the size of the particle, greater will be its surface area.
(3) The effect of pressure on the adsorbate gas
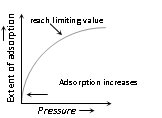
(i) An increase in pressure of the adsorbate gas increases the extent of the adsorption process.
(ii) At the low temperature, the extent of adsorption increases rapidly with the pressure.
(iii) The small range of pressure, extent of adsorption is found to be directly proportional to pressure.
(iv) At very high pressure (closer to the saturation vapour pressure of gas), the adsorption tends to achieve the limiting value.
(4) Effect of temperature
(i) As the adsorption is accompanied by evolution of the heat, so according to the Le-Chatelier's principle, magnitude of the adsorption process should decrease with increase in the temperature.
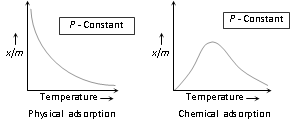
(ii) The relationship between the extent of adsorption and temperature at any constant pressure is called adsorption isobar.
(iii) The physical adsorption isobar shows the decrease in x/m (where m is the mass of the adsorbent and 'x' that of adsorbate) as the temperature rises.
(iv) The isobar of the chemisorption show an increase in starting and then decrease as the temperature rises.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Factors affecting the extent of Adsorption questions? Factors affecting the extent of Adsorption topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Factors affecting the extent of Adsorption related problems. We provide step by step Factors affecting the extent of Adsorption question's answers with 100% plagiarism free content. We prepare quality content and notes for Factors affecting the extent of Adsorption topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours