Factors influencing rate of a reaction are as follows
The Rate of chemical reaction relies on the below stated features
(1) Nature of reactants
(i) Physical state of reactants : This has considerable effect over rate
(ii) Physical size of the reactants : Among the solids, rate increases with decrease in particle size of the solid.
(iii) Chemical nature of the reactants
(a) Reactions involving polar and ionic substances including the proton transfer reactions are generally very fast. Conversely reaction in which bonds is rearranged, or electrons transferred are much slow.
(b) Oxidation-reduction reactions, which include the transfer of electrons, are also slow as compared to the ionic substance.
(c) The substitution much slower in comparison.
(2) Effect of temperature: The rate of chemical reaction usually increases on increasing the temperature. The rate of reaction becomes nearly double or tripled for every 10°C rise in temperature.
The temperature coefficient of the reaction can be defined as the ratio of rate constants at the two temperatures differing by (commonly 25°C and 35°C) 10°C.
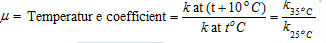
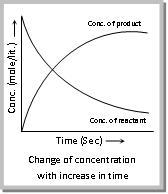
(3) The concentration of reactants can be defined as: The rate of chemical reaction is directly proportional to the concentration of reactants means rate of the reaction decreases with the decrease in the concentration.
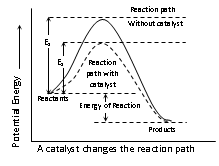
(4) Presence of catalyst: The function of the catalyst is to lower down the activation energy. The greater decrease in the activation energy caused by the catalyst, higher will be reaction rate.
(5) Effect of sunlight: There are several chemical reactions whose rate is influenced by radiations specifically by ultraviolet and visible light. This type of reactions is termed as photochemical reactions. FExamples of which are Photosynthesis, Photography, Photochemical synthesis of compounds Blue printing and many more
Radiant energy starts the chemical reaction by supplying the needed activation energy needed for the reaction.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Factors affecting rate of a reaction questions? Factors affecting rate of a reaction topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Factors affecting rate of a reaction related problems. We provide step by step Factors affecting rate of a reaction question's answers with 100% plagiarism free content. We prepare quality content and notes for Factors affecting rate of a reaction topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours