The Factors affecting electrolytic conductance
In common, conductance of the electrolyte depends upon the factors given below,
(1) Nature of electrolyte : The conductance of the electrolyte depends upon the number of the ions present in the solution. Hence, the greater the number of ions in the solution the greater is the conductance. Number of ions produced by the electrolyte depends upon the nature of it. The strong electrolytes dissociate almost completely into ions in solutions and, therefore, their solutions have the high conductance. On the other hand, the weak electrolytes dissociate to only small extents and provide lesser number of ions. Hence, the solutions of weak electrolytes posses low conductance.
(2) Concentration of the solution : The molar conductance of electrolytic solution varies with the concentration of the electrolyte. Generally, the molar conductance of an electrolyte increases with the decrease in concentration or increase in dilution.
The molar conductance of strong electrolyte ( HCl, KCl, KNO3 )as well as weak electrolytes (CH3COOH, NH4OH) increase with decrease in concentration or increase in dilution. The variation is though different for strong and weak electrolytes.
Variation of the molar conductance with the concentration can be explained on the basis of conducting ability of the ions for weak and strong electrolytes.
For weak electrolytes the variation of 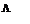 with dilution can be explained on the bases of number of ions in solution. Number of ions furnished by the electrolyte in solution depends upon the degree of dissociation with dilution. When there is increase in the dilution, the degree of dissociation increases and as a result molar conductance increases. Limiting value of the molar conductance
with dilution can be explained on the bases of number of ions in solution. Number of ions furnished by the electrolyte in solution depends upon the degree of dissociation with dilution. When there is increase in the dilution, the degree of dissociation increases and as a result molar conductance increases. Limiting value of the molar conductance 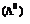 corresponds to the degree of dissociation equal to 1 that is the whole of the electrolyte dissociates.
corresponds to the degree of dissociation equal to 1 that is the whole of the electrolyte dissociates.
Therefore, the degree of dissociation can be calculated at any concentration as,
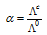
where 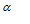 is the degree of dissociation,
is the degree of dissociation,
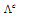 is the molar conductance at the C concentration and
is the molar conductance at the C concentration and
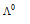 is the molar conductance at the infinite dilution.
is the molar conductance at the infinite dilution.
For strong electrolytes, there is no increase in the number of ions with dilution because strong electrolytes are completely ionized in solution at all concentrations (By the definition). Though, in concentrated solutions of strong electrolytes there are strong forces of attraction between the ions of opposite charges called inter-ionic forces. Because of these inter-ionic forces the conducting ability of ions is less in concentrated solutions. With the dilution, ions become far apart from one another and inter-ionic forces decrease. This results in increase in molar conductivity with the dilution. When the concentration of the solution becomes quite low, the inter-ionic attractions become negligible and molar conductance approaches the limiting value called as molar conductance at the infinite dilution. This value is characteristic of each electrolyte.
(3) Temperature : The conductivity of an electrolyte depends upon the temperature. With increase in the temperature, conductivity of an electrolyte increases.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Factors affecting electrolytic conductance questions? Factors affecting electrolytic conductance topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Factors affecting electrolytic conductance related problems. We provide step by step Factors affecting electrolytic conductance question's answers with 100% plagiarism free content. We prepare quality content and notes for Factors affecting electrolytic conductance topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours