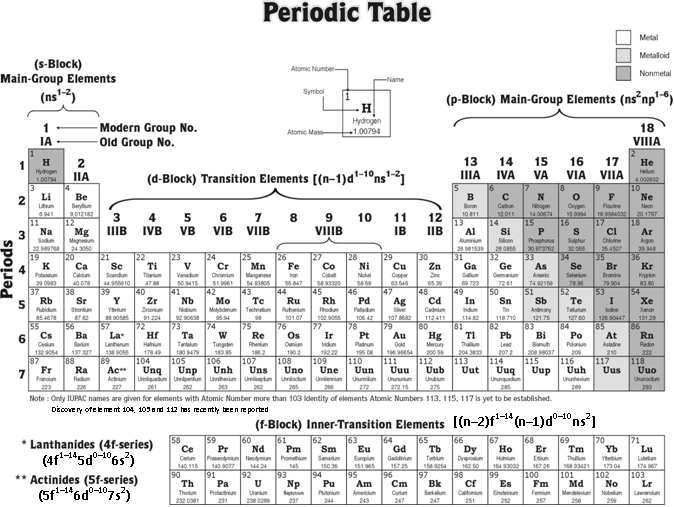
Extended or long form of periodic table
Modern periodic table is also called long form of the periodic table or also called as Bohr's table. In this table, the elements are arranged in order of their increasing atomic number. It consists of 4 blocks (s, p, d and f), 18 groups numbered from 1 to 18 and 7 periods numbered from 1 to 7.
Blocks : The periodic table is divided into four main blocks (s, p, d and f) depending upon the subshell to which the valence electron enters into.
(1) The elements of group 1 and 2 comprise s-Block.
(2) The elements of group 13, 14, 15, 16, 17, 18 comprise p-Block
(3) The elements of group 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 comprise d-Block
(4) The elements of f-Block comprise two horizontal rows placed at bottom of the periodic table to avoid its unnecessary expansion.
The elements of s- and p-blocks are known as normal or representative elements, those of d-block are called transition elements while the f-block elements are termed as inner transition elements.
Groups : The 18 vertical columns are called groups. The elements belonging to a particular group is known as a family and is usually named after the first number. Apart from this some of the groups are given typical names as examplified beneath,
(1) The elements of group 1 are known as Alkali-Metals.
(2) The elements of group 2 are known as Alkaline Earths.
(3) The elements of group 3 are known as Pnicogens.
(4) The elements of group 16 are known as Chalcogens.
(5) The elements of group 17 are known as Halogens.
(6) The elements of group 18 are known as Noble Gases or Aerogens
All these groups are named after the first member of each group.
Periods : The horizontal rows are called periods. There are seven periods in the long form of the periodic table,
(1) Ist period 1H → 2He has 2 elements. It is the most shortest period.
(2) 2nd period (3Li → 10Ne) and 3rd period (11Na → 18Ar) contains 8 elements each. These are short periods.
(3) 4th period (19K → 36Kr) and 5th period (37Rb → 54Ne) contains 18 elements each. These are periods long.
(4) 6th period (55Cs → 86Ra) consists of 32 elements and this period is the longest.
(5) 7th period starting with 87Fr is incomplete and has of 19 elements.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Extended or long form of periodic table questions? Extended or long form of periodic table topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Extended or long form of periodic table related problems. We provide step by step Extended or long form of periodic table question's answers with 100% plagiarism free content. We prepare quality content and notes for Extended or long form of periodic table topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours