Entropy and Entropy change
(1) Definition : Entropy is a thermodynamic state quantity which is a measure of randomness or disorder of the molecules of the system.
Entropy is represented by the symbol S. It is very difficult to define the actual entropy of a system. It is much more convenient to define the change of entropy during a change of state.
The change in entropy of the system may be defined as the integral of all the terms involving heat exchanged (q) divided by the absolute temperature (T) during each infinitesimally small change of the process carried out reversibly at constant temperature.
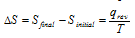
If heat is absorbed, then ΔS=+ve and if heat is evolved from it, then ΔS=-ve
(2) Units of entropy : Since entropy change is expressed by a heat term divided by temperature, it can be expressed in terms of calorie per degree, that is cal deg-1 . In SI units, the entropy is expressed in terms of joule per degree Kelvin, that is JK-1.
(3) Characteristics of entropy: The important characteristics of entropy are summed up below
(i) Entropy is an extensive property. The value of it depends upon the quantity of the substance present in the system.
(ii) Entropy of the system is a state function. It depends upon the state of the variables T,p,V,n.
(iii) The change in entropy in moving from one state to another is independent of the path.
(iv) The change occurring in entropy for the cyclic process is always zero.
(v) The total entropy change of an isolated system is equal to the entropy change of system and entropy change of the surroundings. The sum is termed as entropy change of universe.
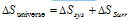
(a) In a reversible process,  and, thus
and, thus
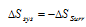
(b) In an irreversible process,  . This means that there is increase in entropy of universe is spontaneous changes.
. This means that there is increase in entropy of universe is spontaneous changes.
(vi) Entropy is a measure of unavailable energy for useful work.
The unavailable energy = Entropy × Temperature
(vii) Entropy, S is related to thermodynamic probability (W) by the relation,
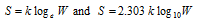
here, k is Boltzmann's constant
(4) Entropy changes in system & surroundings and total entropy change for Exothermic and Endothermic reactions: Heat increases the thermal motion of the atoms or molecules and increases their disorder and hence their entropy. In case of the exothermic process, the heat escapes into surroundings and thus, entropy of surroundings increases on the other hand in case of the endothermic process, the heat enters system
from the surroundings and therefore. The entropy of surroundings decreases.
In general, there will be an overall increase of the total entropy (or disorder) whenever the disorder of surroundings is greater than the decrease in disorder of system. The process will be spontaneous only when there is increase in the total entropy.
(5) Entropy change during phase transition : The change of matter from one state (such as solid, liquid or gas) to another is called phase transition. This type of changes occurs at definite temperature such as melting point (solid to liquid). Boiling point (liquid to vapours) etc, and are accompanied by the absorption or evolution of heat.
When a solid changes into a liquid at its fusion temperature, there is absorption of heat (latent heat). Let ΔHf be the molar heat of fusion. The entropy change can be shown as follows
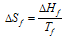
Similarly, if the latent heat of vaporisation and sublimation are denoted by ΔHvap and ΔHsub correspondingly, the entropy of vaporisation and sublimation are given by
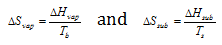
Since ΔHf, ΔHvap and ΔHsub are all positive, these processes are accompanied by increase of entropy and the reverse processes are accompanied by decrease in entropy.
(6) Entropy change for an ideal gas : In going from initial to final state, the entropy change, ΔS for an ideal gas is given by the following relations,
(i) When T and V are two variables,
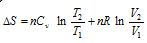 . Assuming Cv is constant
. Assuming Cv is constant
(ii) When T and p are two variables,
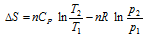 . Assuming Cp is constant
. Assuming Cp is constant
(a) Thus, for an isothermal process (T is constant), 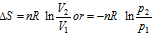
(b) For isobaric process (p constant), 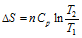
(c) For isochoric process (V constant), 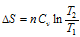
(d) Entropy change during adiabatic expansion : In such process q=0 at all stages. Hence ΔS = 0. Thus, reversible adiabatic processes are called isoentropic process.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Entropy and Entropy change questions? Entropy and Entropy change topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Entropy and Entropy change related problems. We provide step by step Entropy and Entropy change question's answers with 100% plagiarism free content. We prepare quality content and notes for Entropy and Entropy change topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours