Electrovalent bond
The electrovalent bond is formed when the metal atom transfers one or more electrons to the non-metal atom.
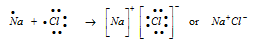
Some of the other examples are: MgCl2, CaCl2, MgO, Na2S, CaH2, AlF3, NaH, KH, , KI, RbCl, NaBr, CaH2 etc.
(1) Conditions for the formation of electrovalent bond
(i) The atom which changes into the cation (+ ive ion) should have 1, 2 or 3 valency electrons. The other atom which changes into the anion (-ve ion) should have 5, 6 or 7 electrons in the valency shell.
(ii) A high difference of electro negativity (about 2) of the two atoms is necessary for the formation of an electrovalent bond. The Electrovalent bond is not possible between the similar atoms.
(iii) There must be overall decrease in energy which means that the energy must be released. For this purpose an atom should have low value of Ionisation potential and the other atom should have high value of electron affinity.
(iv) Higher the lattice energy, the greater will be the case of the forming an ionic compound. The quantity of energy released when free ions combine together to form one mole of a crystal is called lattice energy (U). Lattice energy 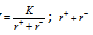 ; is inter nuclear distance.
; is inter nuclear distance.
The energy changes in the formation of ionic compounds from their constituent elements can be studied with the help of a thermochemical cycle called Born Haber cycle.
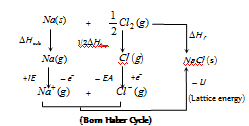
In accordance to Hess's law of constant heat summation, heat of the formation of an ionic solid is net resultant of above changes.
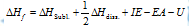
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Electrovalent Bond questions? Electrovalent Bond topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Electrovalent Bond related problems. We provide step by step Electrovalent Bond question's answers with 100% plagiarism free content. We prepare quality content and notes for Electrovalent Bond topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours