The Electronic configurations of the elements
On the basis of electronic configuration principles the electronic configuration of the various elements are given in the table given as follows :
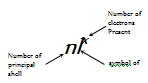
The above method or technique of writing the electronic configurations is much cumbersome. Hence, generally the electronic configuration of an atom of any of the element is simply represented by the notation.
Some of the Unexpected Electronic Configuration
Some of the exceptions are significant though, because they occur with the common elements, particularly chromium and copper.
The Cu has 29 electrons. Its excepted electronic configuration can be given as 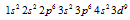 but in actuality the configuration is given as
but in actuality the configuration is given as 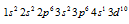 as this configuration is much more stable. Similarly Cr has configuration of
as this configuration is much more stable. Similarly Cr has configuration of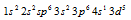 instead of
instead of 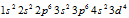 .
.
The factors responsible for the extra stability of the half-filled and the completely filled subshells,
(i) Symmetrical distribution: It is a well known fact that the symmetry leads to the stability. Hence the electronic configuration in which all orbitals of the same subshell are either completely filled or are exactly half filled are much more stable because of the symmetrical distribution of electrons.
(ii) Exchange energy: The electrons with the parallel spins present in the degenerate orbitals have a tendency to exchange their position. The energy released during this type of exchange is known as exchange energy. The number of exchanges which can take place is maximum when the degenerate orbtials (orbitals of the same subshell having equal amount of energy) are exactly half-filled or completely filled. As a result which the exchange energy is maximum and so is the stability.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Electronic configurations of the elements questions? Electronic configurations of the elements topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Electronic configurations of the elements related problems. We provide step by step Electronic configurations of the elements question's answers with 100% plagiarism free content. We prepare quality content and notes for Electronic configurations of the elements topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours