Electron (-1eo)
(1) It was discovered by the scientist J.J. Thomson (1897) and it is negatively charged particle. Electron is a component particle of the cathode rays.
(2) Cathode rays were discovered by the William Crooke's & J.J. Thomson (in the year1880) by making use of a cylindrical hard glass tube fitted with the two metallic electrodes. The tube has a side tube with the stop cock. This tube was called as discharge tube. They passed electricity (10,000V) through the discharge tube at very low pressure (which is 10-2 to10-3 mm Hg. Blue rays were emitted from the cathode. These rays were termed as the Cathode rays.
(3) Properties of Cathode rays are given below
(i) The Cathode rays travel in the straight line and has a straight path.
(ii) Cathode rays produce the mechanical effect, as they can rotate the wheel placed in the path they are travelling through.
(iii) Cathode rays comprise of negatively charged particles called as electron.
(iv) Cathode rays travel with the high speed approaching the speed that of light (ranging between 10-9 to 10-11 cm/sec)
(v) Cathode rays can create fluorescence.
(vi) The Cathode rays heat the particular object on which they are falling due to the transfer of kinetic energy to that object.
(vii) When the cathode rays fall on the solids such as Cu, X-rays are produced from them.
(viii) Cathode rays acquire the ionizing power that is they ionize the gas through which they are passing.
(ix) The cathode rays produce the phenomenon of scintillation on the photographic plates.
(x) They can penetrate and pass through the thin metallic sheets.
(xi) The nature of these rays does not rely upon the nature of gas or cathode material used in the discharge tube.
(xii) The e/m (charge to mass ratio) for the cathode rays was found to be the same as that for the e- (-1.76 * 108 coulomb per gram coloumb per gm). Therefore, the cathode rays are the stream of electrons.
(xiii) According to the Einstein's theory of the relativity, mass of an electron in the motion is given by, 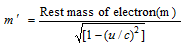
Where u = velocity of electron, c= velocity of light.
When u=c than mass of moving electron = 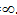
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Electron questions? Electron topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Electron related problems. We provide step by step Electron question's answers with 100% plagiarism free content. We prepare quality content and notes for Electron topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours