Electron affinity
Those atoms whose nuclear forces are not completely screened by electronic shells, gives attraction for electrons. Such type of atoms capture electrons if these are available within their effective fields to neutralize the electrostatic forces of the nucleus. The energy is always liberated each time there is a force of attraction offered by an atom or ion, and this energy is known as electron affinity. This can be defined as, ''the energy released when an extra electron is added to a neutral gaseous atom''.
When first electron is added in a neutral atom then some energy is released that is called first electron affinity but in case of second electron affinity energy will be absorbed due to electronic repulsion. The example of it is given as follows
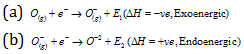
Therefore, higher the energy released in the process of taking up the extra electron, higher will be the electron affinity. The higher value of electron affinity of an atom, more will be its tendency to change into anion. It is quite difficult to determine the electron affinity experimentally. The values have been obtained on the basis of thermodynamic concepts. It can be expressed in electron volts. The values of inert gases are supposed to be zero because they have stable ns2p6 configuration and unable to accept any electron. The values for the alkali metals are comes between zero and one.
The electron affinities of Be, Mg and zero since they have complete ns2 configuration which cannot accommodate extra electron. Likewise, the values for N and P are very low because they also have completely half-filled p orbitals (ns2p3) and are more stable.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Electron affinity questions? Electron affinity topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Electron affinity related problems. We provide step by step Electron affinity question's answers with 100% plagiarism free content. We prepare quality content and notes for Electron affinity topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours