Electrode potentials (Eo):
3d-series of transition elements and their ions are involved in Standard electrode potentials of some half-cells aqueous solution are given in table below,
Standard electrode potentials for 3d-elements
|
Elements
|
Ion
|
Electrode reaction
|
E°/ volt
|
Sc
|
Sc3+
|
Sc3++ 3e- → Sc
|
- 2.10
|
|
Ti
|
Ti2+
|
Ti2++ 2e- → Ti
|
- 1.60
|
|
V
|
V2+
|
V2++ 2e- → V
|
- 1.20
|
|
Cr
|
Cr3+
|
Cr3+ + 3e- → Cr
|
- 0.71
|
|
Mn
|
Mn2+
|
Mn2++ 2e- → Mn
|
- 1.18
|
|
Fe
|
Fe2+
|
Fe2+ + 2e- → Fe
|
- 0.44
|
|
Co
|
Co2+
|
Co2+ + 2e- → Co
|
- 0.28
|
|
Ni
|
Ni2+
|
Ni2+ + 2e- → Ni
|
- 0.24
|
|
Cu
|
Cu2+
|
Cu2+ + 2e- → Cu
|
+ 0.34
|
|
Zn
|
Zn2+
|
Zn2+ + 2e- → Zn
|
- 0.76
|
A negative value of E° for the first series of transition elements except for Cu2+/ Cu will be implies the following things:
(a) From dilute acids these metals should liberate hydrogen that is the reactions that was given below,
M + 2H+ → M2+ + H2 (g); 2M + 6H+ → 2M3+ + 3H2(g)
are favorable in the other direction. In fact practice though, many of these metals react with the dilute acids extremely slowly. A few of these metals will be coated with the thin protective layer of oxide element. This category an oxide layer avoids the metal to react additionally.
(b) There is no regular trend in the E° values. The metals should act as good reducing agents. This is because of irregular variation in the sublimation and ionisation energies across the series.
Associative stabilities of transition metal ions in various oxidation states in aqueous medium could be predicted from an electrode potential data. To demonstrate this, now let us assume the following,
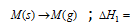 Enthalpy of sublimation, DHsub
Enthalpy of sublimation, DHsub
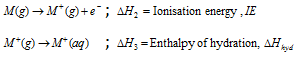
Adding these equations one than we gets,
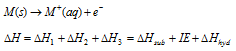
Here the ΔH should represents the enthalpy modified that needs to bring the solid metal M to the monovalent ion in aqueous medium, M+(aq).
The reaction, M(s) → M+(aq) +e-, will be favourable just only when ΔH is negative. Many negative value is the value of ΔH, more favourable will be the created of that cation from the metal. Moreover, the oxidation state for ΔH value is more negative will be stable in the solution.
Electrode potential for a Mn+/M half-cell is a measure of the tendency for the reaction that was given below
Mn+(aq) + ne- → M (s)
Therefore, by the above equation this reduction reaction will take place if the electrode potential for Mn+/M half- cell is positive. The reverse reaction is ghiven below,
M(s) →Mn+(aq) + ne-
Including the formation of Mn+(aq) will be occur if the electrode potential is negative, which is the tendency for the creation of Mn+(aq) from the metal M will be more if the corresponding E° value is more negative. Or we could say in which the oxidation state for that E° value is more negative (or less positive) will be more stable by the solution.
Here the standard electrode potential (E°) values can be used in the predicting the relative stabilities of different oxidation states in aqueous solutions when an elements exists in more than one oxidation states, the below stated rule is found useful. The oxidation state of a cation for the given equation 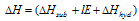 or E° is more negative (for less positive) will be more stable.
or E° is more negative (for less positive) will be more stable.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Electrode Potential questions? Electrode Potential topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Electrode Potential related problems. We provide step by step Electrode Potential question's answers with 100% plagiarism free content. We prepare quality content and notes for Electrode Potential topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours