Effects of hydrogen bonding
The Hydrogen bond helps in the explaining abnormal physical properties in the number of cases. Some of the properties affected by the H-bond are written below,
(1) Dissociation : In the aqueous solution, the hydrogen fluoride dissociates and gives the difluoride ion 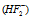 instead of the fluoride ion (F-) . This is cause of the H-bonding in HF. This explains existence of the KHF2. H-bond formed is generally longer than the covalent bond present in the molecule (for example in H2O, O-H bond = 0.99 Å but H-bond = 1.77 Å).
instead of the fluoride ion (F-) . This is cause of the H-bonding in HF. This explains existence of the KHF2. H-bond formed is generally longer than the covalent bond present in the molecule (for example in H2O, O-H bond = 0.99 Å but H-bond = 1.77 Å).
(2) Association : The molecules of the carboxylic acids exist as dimers cause of the hydrogen bonding. The molecular masses of such type of compounds are found to be double than those which are calculated from their simple formulae. For instance, molecular mass of acetic acid is found to be 120.
(3) High melting and boiling point: Compounds which are having hydrogen bonding show abnormally high melting and boiling points.
The high melting points and boiling points of the compounds H2O, HF and NH3 having the hydrogen bonds is due to the fact that some extra energy is required to break these bonds.
(4) Solubility : Compound which can form the hydrogen bonds with the covalent molecules are soluble in such type of solvents. For instance, lower alcohols are soluble in water because of hydrogen bonding which can take place between water and alcohol molecules as given below,
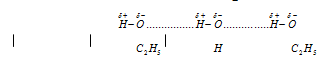
Intermolecular hydrogen bonding increases the solubility of the compound in water as, the intramolecular hydrogen bonding decrease
(5) As the compounds involving hydrogen bonding between different molecules (intermolecular hydrogen bonding) posses higher boiling points, so they are less volatile in nature.
(6) The substances which contain the hydrogen bonding have higher viscosity and the high surface tension.
(7) Explanation of the lower density of ice than water and maximum the density of water at 277K : In case of the solid ice, hydrogen bonding gives rise to the cage like structure of water molecules as shown in the figure drawn below. As the matter of fact, each water molecule is linked tetrahedrally to the four other water molecules. Cause to this structure ice has lower density than the density of water at 273K That is the reason why ice floats on water. On heating, hydrogen bonds begin collapsing, obviously the molecules are not so closely packed to each other as they are in the liquid state and hence the molecules begin coming together resulting in the decrease of volume and hence increase of density. This goes on up to the temperature277K. After 277 K, increase in the volume due to expansion of the liquid water becomes much more than the decrease in the volume due to breaking of H-bonds. Therefore, after 277K, there is net increase of the volume on heating which means decrease in the density. Therefore density of water is maximum 277K.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Effects of Hydrogen Bonding questions? Effects of Hydrogen Bonding topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Effects of Hydrogen Bonding related problems. We provide step by step Effects of Hydrogen Bonding question's answers with 100% plagiarism free content. We prepare quality content and notes for Effects of Hydrogen Bonding topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours