Dual nature of the electron
(1) In the year 1924, the French physicist, Louis de Broglie suggested that if the light has particle and wave like nature both, the similar duality should be true for the matter. Therefore an electron, behaves both as a material particle and as a wave.
(2) This presented a new wave mechanical theory of the matter. In accordance to this theory, small particles like electrons when in motion it possesses wave properties.
(3) According to the de-broglie, the wavelength associated with the particle of mass m, moving with velocity v can be given by the relation
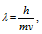
here h = Planck's constant.
(4) This can be derived as shown below according to Planck's equation,
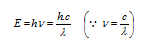
the energy of photon (on basis of Einstein's mass energy relationship),
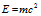
Equating both the equations
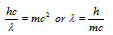
which is same as the de-Broglie relation.
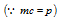
(5) This was experimentally verified by the Davisson and Germer by observing the effects of diffraction with an electron beam. Let the electron is accelerated with the potential of V than the Kinetic energy can be given as
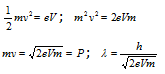
(6) If the Bohr's theory is related with the de-Broglie's equation then wave length of an electron can be determined in the bohr's orbit and relate it with circumference and multiply with the whole number
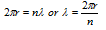
From the de-Broglie equation, 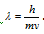 .
.
Therefore or 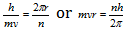
(7) The de-Broglie equation is applicable to all the material objects but it has significance only in the case of microscopic particles. As, we come across macroscopic objects in our day to day life; de-broglie relationship dosent has significance in everyday life.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Dual nature of the electron questions? Dual nature of the electron topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Dual nature of the electron related problems. We provide step by step Dual nature of the electron question's answers with 100% plagiarism free content. We prepare quality content and notes for Dual nature of the electron topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours