Dipole moment
The product of magnitude of the negative or positive charge (q) and the distance (d) between their centres of positive and negative charges is known as dipole moment.
μ = Electric charge * bond length
As q is in the order of 10-10 esu and d is in the order of 10-8 cm, m is in the order of 10-18 esu cm. The dipole moment can be measured in "Debye" (D) unit. 1 D = 10-18 esu cm = 3.33 * 10-30 coulomb metre (In S.I. unit).
Dipole moment is indicated by the arrow having the symbol of ( ) pointing towards negative end. The Dipole moment has both magnitude and direction and hence it is a vector quantity.
) pointing towards negative end. The Dipole moment has both magnitude and direction and hence it is a vector quantity.
Symmetrical polyatomic molecules are not polar so they do not have any value of dipole moment.
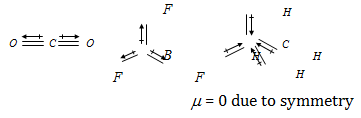
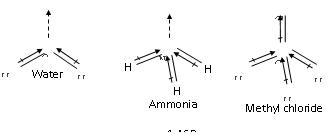
Unsymmetrical polyatomic molecules always have the net value of dipole moment, hence such molecules are polar in nature. H2O, CH3Cl, NH3, etc are polar molecules as they have some positive values of dipole moments.
μ ≠ 0 due to unsymmetry
(1) The dipole moment is a significant factor in determining the geometry of molecules.
: 3.1 Molecular geometry and dipole moment
|
General formula
|
Molecular geometry
|
Dipole moment
|
Example
|
|
AX
|
Linear
|
May be non zero
|
HF, HCl
|
| AX2
|
Linear
Bent or V-shape
|
Zero
Non zero
|
CO2, CS2
H2O, NO2
|
| AX3
|
Triangular planar
Pyramidal
T-shape
|
Zero
Non zero
Non zero
|
BF3
NH3, PCl3
ClF3
|
| AX4
|
Tetrahedral
Square planar
See saw
|
Zero
Zero
Non zero
|
CH4, CCl4
XeF4
SF4, TeCl4
|
| AX5
|
Trigonal bipyramidal
Square pyramidal
|
Zero
Non zero
|
PCl5
BrCl5
|
| AX6
|
Octahedral
Distorted octahedral
|
Zero
Non zero
|
SF6
XeF6
|
| AX7
|
Pentagonal bipyramidal
|
Zero
|
IF7
|
(2) Each and every ionic compound possessing some percentage of the covalent character according to the Fajan's rule. The percentage of ionic character in the compound having some covalent character can be measured by the following equation.
The % ionic character  .
.
(3) The trans isomer commonly possesses either zero dipole moment or very low value in the comparison to cis-form 
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Dipole Moment questions? Dipole Moment topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Dipole Moment related problems. We provide step by step Dipole Moment question's answers with 100% plagiarism free content. We prepare quality content and notes for Dipole Moment topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours