Hydrides : Hydrogen forms binary hydrides of the type MHx or MmHn with
(a) All important group elements except noble gases and probably indium and thallium.
(b) All the lanthanoids and actinoids.
(c) The transition metals (Zr, Sc, Tc, Y, La, Ac, Hf and to a lesser extent Cr, V, Cu Nb, Ta, and Zn). In group 6 only Cr forms hydride (CrH).
The Hydrides can be classified into three basic categories.
(i) Saline or ionic hydrides : Most of the s-block metals form this type of hydrides. They are non-conducting, non-volatile, crystalline solids. Although, BeH2 and MgH2 posses covalent polymeric structure. These ionic hydrides posses rock-salt structure. The thermal stability of 1st and 2nd group hydrides are in the order;
LiH > NaH > KH > RbH > CsH
CaH2 > SrH2 > BaH2
BeH2, MgH2 and LiH posses important covalent character.
Electrolysis of solution of saline hydride in molten alkali halide produces H2 at anode. Saline hydrides react explosively with water.
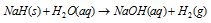
The fire so produced cannot be extinguished by CO2 as it gets reduced by the hot metal hydride. Only the sand is useful, as it is a solid in state.
Alkali metal hydrides are used for making LiAlH4, NaBH4 etc. Alkali metal hydrides are also used for the removal of last traces of water from organic compounds.
(ii) Metallic or interstitial hydrides : Elements of the groups 3, 4, 5 (which is d-block) and f-block elements form the metallic hydrides. In the group number 6, only Cr forms the hydride (CrH). The metals of group number 7, 8, 9 do not form hydrides. This area of periodic table from group 7 to group 9 is known as hydride gap. Examples of the hydrides of group 3 to 5 are, ScH2, YH2, LaH2, LaH3, TiH2, ZrH2, HfH2, VH, VH2, NbH, NbH2, TaH
The f-block metals form hydrides of limiting compositions of MH2 and MH3. All these hydrides are non-stoichiometric with variable composition e.g.,
 .
.
Almost all these hydrides are good conductors of electricity in solid state.
The metallic hydrides can be taken in use to store hydrogen especially in cars working on fuel cells.
(iii) Molecular or covalent hydrides : Hydrogen form molecular compounds with p-block elements (B, C, N, O, F; Si, P, S, Cl; Ga, Ge, As, Sb, Br; In, Sn, Sb, Te, I; Tl, Pb, At). general examples of such hydrides are CH4, NH3, H2O, HF etc. The stability of these hydrides decreases down the group. For instance, NH3 > PH3 > AsH3 > SbH3 > BiH3. In a period the stability increases with increasing electronegativity. For example, CH4 < NH3 < H2O < HF. Molecular hydrides are classified as electron precise electron rich, and electron deficient hydrides.
(a) Electron rich molecular hydrides : These hydrides have one or more lone pairs of electrons around the central more electronegative element. The example is given as
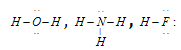
(b) Electron precise molecular hydrides : Elements of group 14 form such hydrides. The bond length increases on moving down the group. A general example of electron precise molecular hydrides is CH4.
(c) Electron deficient molecular hydrides : These hydrides have lesser number of electrons than that required for writing the conventional Lewis structure. A general example of such molecular hydride is diborane, B2H6.
(d) Systematic names of molecular hydrides : The systematic names of these hydrides are obtained from the name of the element and the suffix -ane. For example,
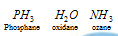
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Different forms of Hydrogen - Hydrides questions? Different forms of Hydrogen - Hydrides topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Different forms of Hydrogen - Hydrides related problems. We provide step by step Different forms of Hydrogen - Hydrides question's answers with 100% plagiarism free content. We prepare quality content and notes for Different forms of Hydrogen - Hydrides topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours