Methods for determination of the order of a reaction
(1) Integration method (commonly known as Hit and Trial method)
(i) The method can be used with various sets of a, x and t with integrated rate equations.
(ii) The value of k is determined and checked for all sets of a, x and t.
(iii) If the value of k is constant, the used equation gives the order of reaction.
(iv) If all the reactants are at the same molar concentration, the kinetic equations are given as follows:

(2) Half-life method : This method is employed only when the rate law involved only one concentration term.
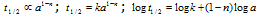
A plotted graph of log t1/2 vs log a gives a straight line with slope (1-n), determining the slope we can find the order n. If half-life at different concentration is given then,
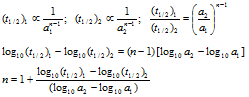
The above relation can be used to determine order of reaction n
Plots of half-lives Vs concentrations (t1/2 µ a1-n)
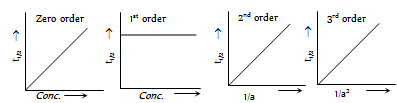
(3) Graphical method : A graphical method based on the respective rate laws, can also be used.
(i) If the plot of log(a-x) Vs t is a straight line, the reaction follows first order.
(ii) If the plot of 1/(a-x) Vs t is a straight line, the reaction follows second order.
(iii) If the plot of 1/(a-x)2 Vs t is a straight line, the reaction follows third order.
(iv) In general, for a reaction of nth order, a graph of 1/(a-x)n-1 Vs t must be a straight line.
Plots from integrated rate equations
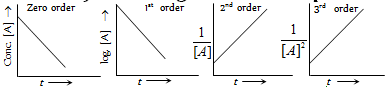
Plots of rate Vs concentrations [Rate = k(conc.)n ]
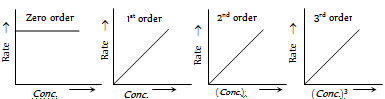
(4) Van't Haff differential method : The rate of reaction varies as the nth power of the concentration Where 'n' is the order of the reaction. Therefore for two different initial concentrations C1 and C2 equation, may be written in the form,
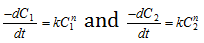
Taking logarithms,
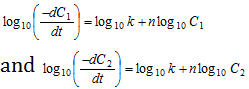
Subtracting equation (ii) from (i),
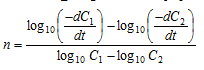 .....(iii)
.....(iii)
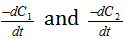 are determined from concentration Vs time graphs and the value of n can be determined.
are determined from concentration Vs time graphs and the value of n can be determined.
(5) Ostwald's isolation method (Initial rate method)
This method can be used irrespective of the number of reactants involved e.g., consider the reaction, 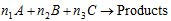 .
.
This method consists in finding the initial rate of the reaction taking known concentrations of the different reactants (such as A, B, C).
Suppose it is observed as follows,
(i) Keeping the concentrations of B and C constant, if the concentration of A is doubled, the rate of the reaction becomes four times. This means that, Rate i.e., order with respect to A is 2
(ii) Keeping the concentrations of A and C constant, if concentration of B is doubled, the rate of reaction is also doubled. This means that, Rate µ [B] i.e., order with respect to B is 1
(iii) Keeping the concentrations of A and B constant, if the concentration of C is doubled, the rate of reaction remains without any affect. This means that rate is independent of the concentration of C i.e., order with respect to C is zero. Hence the overall rate law expression will be, Rate = k[A]2 [B] [C]0
Overall order of reaction = 2 + 1 + 0 = 3.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Determination of the order of a reaction questions? Determination of the order of a reaction topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Determination of the order of a reaction related problems. We provide step by step Determination of the order of a reaction question's answers with 100% plagiarism free content. We prepare quality content and notes for Determination of the order of a reaction topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours