The methods of determination of the equivalent mass
(i) Hydrogen displacement method : The mass of metal which displaces 11200 ml of hydrogen at NTP from an acid, alkali or alcohol is the equivalent mass of the metal.
(a) Equivalent mass of metal

(b) Equivalent mass of metal
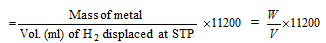
This techniques is very much useful for metals which can displace hydrogen from acids or can combine with hydrogen ( Mg, Zn, Na, Ca etc).
(ii) Oxide formation method: The mass of the element which combines with 8 grams of oxygen is the equivalent mass of the element.
(a) Equivalent mass of metal = 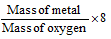
(b) Equivalent mass of metal
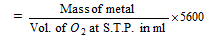
(iii) Chloride formation method: The mass of an element which reacts with the 35.5 gm of chlorine is the equivalent mass of that element.
(a) Equivalent mass of metal
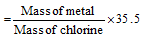
(b) Equivalent mass of metal
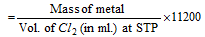
(iv) Neutralization method : (For the acids and bases).
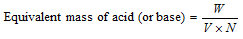
Where ,
W = Mass of acid or base in gm.,
V = Vol. of base or acid in litre needed for neutralization
N is Normality of base or acid
(v) Metal displacement method: This is based on the fact that one gm of the equivalent of a more electropositive metal displaces one gm equivalent of a less electropositive metal from its salt solution.
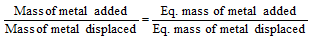
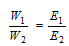
(vi) Electrolytic method : The quantity of substance which reacts at electrode when 1 faraday of electricity is passed is equal to the gram equivalent mass of it.
Gram equivalent mass = Electrochemical equivalent * 96500
The ratio of masses of two metals deposited by the same quantity of electricity will be in the ratio of their equivalent masses respectively.
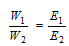
(vii) Double decomposition method
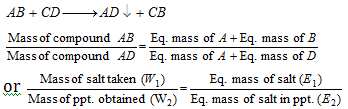
(viii) Conversion method: When one compound of a metal is converted to the another compound of same metal, then we can say that
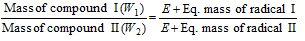
(E = Eq. mass of the metal)
(ix) Volatile chloride method
Valency of metal 
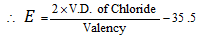
(x) Silver salt method (This is for organic acids)
Equivalent Mass of acid = 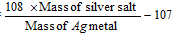
Molecular mass of acid = Equivalent mass of acid * Basicity
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Determination of the equivalent mass questions? Determination of the equivalent mass topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Determination of the equivalent mass related problems. We provide step by step Determination of the equivalent mass question's answers with 100% plagiarism free content. We prepare quality content and notes for Determination of the equivalent mass topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours