Determination of hybridisation at different carbon atoms : It can be done by two methods,
(i) First method : In that process hybridisation can be know by the number of bonds present on that particular atom
|
Number of p- bond/s
|
0
|
1
|
2
|
|
Type of hybridisation
|
sp3
|
sp2
|
sp
|
Examples :
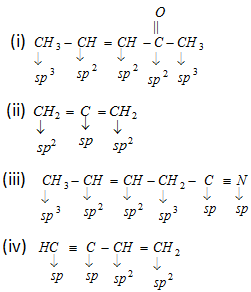
- In diamond carbon is sp3 hybridised and in graphite carbon is sp2 hybridised.
(ii) Second method : (Electron pair process)
ep = bp + lp; where ep = electron pair present in hybrid orbitals , bp = bond pair shown in hybrid orbitals
Number of bp = Number of molecules attached to the central atom of the species
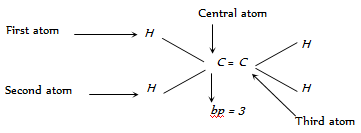
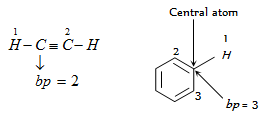
Number of lp's can be calculated as given,
(a) If carbon has - bonds or positive charge or odd electron, than lp on carbon will be zero.
(b) If carbon has negative charge, then lp will be same to one.
Number of electron pairs (ep) defines the type of hybridisation as follows,
|
Ep
|
2
|
3
|
4
|
5
|
6
|
|
Type of hybridisation
|
sp
|
sp2
|
sp3
|
sp3d
|
sp3d2
|
Example :
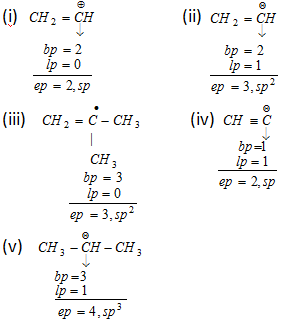
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Determination of hybridisation at different carbon atoms questions? Determination of hybridisation at different carbon atoms topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Determination of hybridisation at different carbon atoms related problems. We provide step by step Determination of hybridisation at different carbon atoms question's answers with 100% plagiarism free content. We prepare quality content and notes for Determination of hybridisation at different carbon atoms topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours