Dalton's law of partial pressures
(1) In accordance to this law, When the two or more gases, which do not react chemically are kept in a closed vessel, total pressure exerted by the mixture is equal to the sum of the partial pressures of individual gases.
Thus, Ptotal = P1 +P2 + P3 + .........
Here P1, P2, P3..... are the partial pressures of the gas number 1, 2, 3 and so on
(2) The Partial pressure is the pressure exerted by the gas when it is present alone in same container and at the same temperature.
Partial pressure of a gas 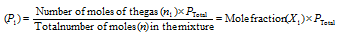
(3) If the number of gases having the volume of V1, V2, V3..... at pressure P1, P2, P3..... are mixed together in the container of the volume denoted by V, thus,
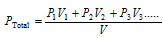
or
or 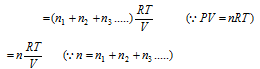
(4) Applications of it: This law is used in calculation of the following relationships,
(i) The Mole fraction of gas (X1) in the mixture of gas 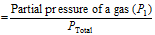
(ii) % of a gas in mixture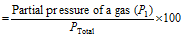
(iii) Pressure of the dry gas collected over water : When the gas is collected over water, it becomes moist because of the water vapour which exerts its own partial pressure at the same temperature of gas. This partial pressure of the water vapours is called as aqueous tension. Therefore,
Pdry gas = Pmoist gas or Ptotal - Pwater vapour
or Pdry gas = Pmoist gas - Aqueous tension (Aqueous tension is directly proportional to absolute temperature)
(iv) Relative humidity (RH) at a given temperature is given by,

(5) Limitations : This law is applicable only when the component gases in the mixture do not react with each other. For example, N2 and O2, CO and CO2, N2 and Cl2, CO and N2 etc. But this law is not applicable to gases which combine chemically. For example, H2 and Cl2, CO and Cl2, NH3, HBr and HCl, NO and O2 etc.
(6) Another law, which is really equivalent to the law of partial pressures and related to the partial volumes of gases is known as Law of partial volumes given by Amagat. In accordance to this law, When the two or more gases, which do not react chemically are kept in the closed vessel, the total volume exerted by mixture is equal to the sum of the partial volumes of the individual gases.
Therefore, VTotal = V1 + V2 +V3+ .....
Here V1, V2,V3, are the partial volumes of gas number 1, 2, 3 and so on
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Dalton's law of partial pressure questions? Dalton's law of partial pressure topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Dalton's law of partial pressure related problems. We provide step by step Dalton's law of partial pressure question's answers with 100% plagiarism free content. We prepare quality content and notes for Dalton's law of partial pressure topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours