Consequences of Metal excess defects
The crystals with metal excess defects are generally coloured due to the presence of free electrons in them.
The crystals with metal excess defects conduct electricity due to the presence of free electrons and are the semiconductors. As electric transport is majorly by the excess electrons, these are called n-type (n for negative) semiconductor.The crystals with the metal excess defects are usually paramagnetic in nature due to the presence of unpaired electrons at lattice sites.
When the crystal is irradiated with the white light, trapped electron absorbs some component of the white light for excitation from ground state to the excited state. This gives rise to the colour. Such type of points are called F-centres. (German word Farbe which means colour) such excess ions are accompanied by the positive ion vacancies. These vacancies serve to trap the holes in the same manner as the anion vacancies trapped electrons. The colour centres therefore produced are called V-centres.
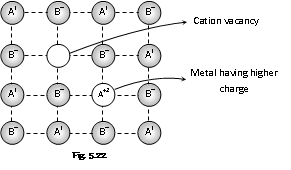
(c) Metal deficiency defect by the cation vacancy : In this the cation is missing from its lattice site. To maintain the electrical neutrality, one of the nearest metal ion takes two positive charge. This type of defect occurs in the compounds where metal can posses variable valency. For example Transition metal compounds such as FeO, NiO, FeS etc.
(d) By having extra anion occupying interstitial site : In this, an extra anion is present in the interstitial position. Extra negative charge is balanced by one extra positive charge on adjacent metal ion. Since the anions are generally larger it could not occupy an interstitial site. Hence, this structure has theoretical possibility only. No example of it is known so far.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Consequences of Metal excess defects questions? Consequences of Metal excess defects topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Consequences of Metal excess defects related problems. We provide step by step Consequences of Metal excess defects question's answers with 100% plagiarism free content. We prepare quality content and notes for Consequences of Metal excess defects topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours