Compounds of zinc
(1) Zinc oxide (Zinc white or Chinese white), ZnO : It is obtained by burning zinc in air or by heating zinc carbonate or zinc nitrate.
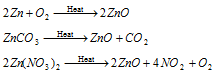
It is a white powder but becomes yellow on heating and again white on cooling.
It is insoluble in water and is very light and hence commonly called as philosopher's wool.
It is amphoteric in nature.
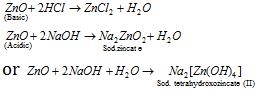
It is reduced by carbon and H2 both and is used as a white paint
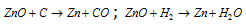
(2) Zinc chloride, ZnCl2 : It is obtained when Zn metal, ZnO or ZnCO3 is treated with dil. HCl. It crystallizes as ZnCl2.2H2O and becomes anhydrous on heating. ZnCl2 is highly deliquescent and is highly soluble in H2O and also readily dissolves in organic solvents such as alcohol, acetone, ether etc. its aqueous solution is acidic due to hydrolysis.
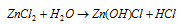
Anhydrous ZnCl2 is used as a Lewis acid catalyst in organic reactions. This is mixed with the moist zinc oxide, which is used for filling the teeth and its solution is used for preserving the timber. Anhydrous ZnCl2 used as a Lucas reagent with concentrated HCl.
(3) Zinc sulphide, ZnS : It is a white solid. It is soluble in dililute HCl and thus does not get precipitated by H2S in the acidic medium.
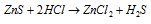 .
.
It is a constituent of lithopone (ZnS + BaSO4)
(4) Zinc sulphate, ZnSO4.7H2O : It is commonly known as white vitriol and is obtained by the action of dil.H2SO4 on zinc metal, ZnO or ZnCO3. On heating, it loses six molecules of water of crystallization first at the temperature 373 K. At the temperature of 723 K, it becomes anhydrous and on additional heating, it decomposes.
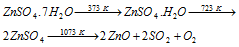
It is used to prepare lithopone (BaSO4 + ZnS), a white paint and also in galvanising iron.
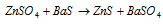
It is also used as an eye lotion.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Compounds of Zinc questions? Compounds of Zinc topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Compounds of Zinc related problems. We provide step by step Compounds of Zinc question's answers with 100% plagiarism free content. We prepare quality content and notes for Compounds of Zinc topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours