Sodium hydroxide NaOH (Caustic soda)
Preparation
(a) Gossage process :
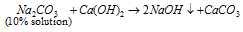
(b) The electrolytic method : Caustic soda is manufactured by electrolysis of the concentrated solution of NaCl
At anode: Cl- discharged; At cathode: Na+ discharged
(c) Castner - Kellener cell (Mercury cathode process): NaOH obtained by electrolysis of aqueous solution of brine. The cell consists of rectangular iron tank divided into three compartments.
Outer compartment - Brine solution is electrolysed; Central compartment - 2% NaOH solution and H2
Properties : White crystalline solid, highly soluble in water, It is sparingly soluble in alcohol.
(a) Reaction with salt :
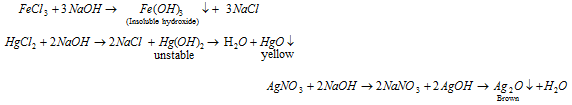
Zn, Al, Sb, Pb, Sn and As forms insoluble hydroxide which dissolve in excess of NaOH (amphoteric hydroxide).
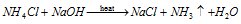
(b) Reaction with halogens :
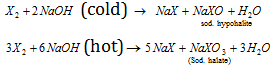
(X = Cl, Br, I)
(c) Reaction with metals : Weakly electropositive metals like Zn, Al and Sn etc.
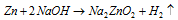
(d) Reaction with sand, SiO2 :
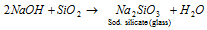
(e) Reaction with CO:
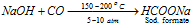
NaOH Breaks down proteins of the skin flesh to the pasty mass, thus it is commonly called as caustic soda.
Caustic property : sodium hydroxide breaks down the proteins of the skin flesh to a pasty mass, therefore, it is commonly known as caustic soda.
Uses : Sodium hydroxide is used :
(a) in the manufacture of sodium metal, soap (from oils and fats), rayon, paper, dyes and drugs,
(b) for mercurinzing cotton to make cloth unshrinkable and
(c) as a reagent in the laboratory.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Compounds of Sodium - Sodium hydroxide questions? Compounds of Sodium - Sodium hydroxide topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Compounds of Sodium - Sodium hydroxide related problems. We provide step by step Compounds of Sodium - Sodium hydroxide question's answers with 100% plagiarism free content. We prepare quality content and notes for Compounds of Sodium - Sodium hydroxide topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours