Compounds of Silver:
(1) Silver oxide (Ag2O): It is unstable and decomposes into Ag and O2 on slow heating.
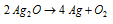
(2) Silver halides (AgF, AgCl, AgBr and Agl) : Only AgF is soluble in H2O. AgCl is insoluble in H2O but dissolves in NH4OH, Na2S2O3 and KCN solutions. AgBr is partly soluble whereas AgI is completely insoluble in NH4OH. Except AgF, all the remaining three silver halides are photosensitive.
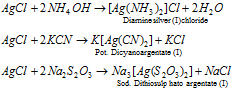
(3) Silver nitrate (AgNO3) : Silver nitrate (AgNO3) is called lunar caustic silver nitrate on heating above its melting point (485 K) decomposes to silver nitrite but on heating to red heat gives silver.

When treated with alkali, AgNO3 forms silver oxide which in case of NH4OH dissolves to form complex ion.
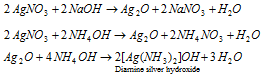
AgNO3 reacts with iodine in two ways
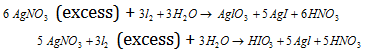
In contact with organic matter (skin, cloth, paper etc.) AgNO3 is reduced to metallic silver (black)
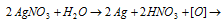 oxidises organic matter
oxidises organic matter
AgNO3 gives different coloured ppt. with different anions like 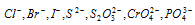 etc.
etc.
AgNO3 can be used in preparation of the ink and hair dyes.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Compounds of Silver questions? Compounds of Silver topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Compounds of Silver related problems. We provide step by step Compounds of Silver question's answers with 100% plagiarism free content. We prepare quality content and notes for Compounds of Silver topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours