Compounds of mercury:
(A) Mercuric oxide, HgO: the first compound of mercury is Mercuric oxide, HgO. It is acquired as a red solid by heating mercury in air or oxygen for very long time at 673 K
Equation:
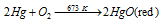
or via heating the mercuric nitrate alone or in existence of the Hg (mercury)
Equation:
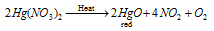
While NaOH is added to a solution of HgCl2, yellow precipitate of HgO are acquired.
Reaction is as follow:
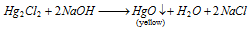
Red and yellow types of HgO are different only in their particle size. On heating to the temperature that is of 673 K, yellow form changes to red form.
the equation for this is as follow:
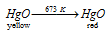
It is taken as a mild antiseptic in the ointments or in the oil paints.
(B) Mercuric chloride, HgCl2 : It is acquired by treating Hg along with Cl2 or via heating a mixture of NaCl and HgSO4 in existence of small amount of Mn2O (that oxidises any Hg(I) salts that is made during the reaction).
Reaction is as follow:
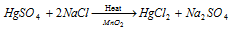
It is a white crystalline solid and is generally termed as corrosive sublimate. It is the covalent compound because it dissolves in organic solvents such as ether and ethanol.
It is very poisonous and reason for death. The most excellent antidote of it is white of an egg.
It is while treated along with the stannous chloride, first gets reduced to white precipitate of mercurous chloride and after that to mercury (black).
Explained as:
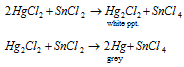
With ammonia it provides a white ppt. termed as infusible white precipitate.
That is explained below in the equation:
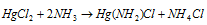
A dilute solution of HgCl2 is employed like an antiseptic.
(C) Mercuric iodide, HgI2 : It is acquired while a needed amount of KI solution is added to a solution of HgCl2.
Reaction for above is as follow:
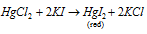
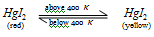
Beneath 400 K, Hgl2 is red but higher than the temperature that is of 400 K, it turns yellow
Hgl2 simply dissolves in the excess of KI solution to make the (HgI4)2- complex ion.
Explained like this:
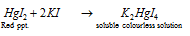
An alkaline solution of K2[HgI4] is termed as Nessler's reagent and is employed to test NH4+ ions.
It provides a brown ppt. of NH2-Hg-O-Hg-I (Iodide of Millon's base) along with NH4+ ions.
Equation for the above process is as follow:
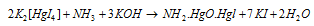
This is employed in ointments that are used for treating the skin infections.
(D) Mercurous chloride, Hg2Cl2 : It is acquired like :
(a) 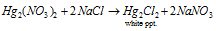
(b) 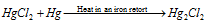 (condenses on cooling)
(condenses on cooling)
It is purified by sublimation.
Mercurous chloride is as well called calomel. It is a white powder that is insoluble in H2O. It decomposes to provide HgCl2 and Hg , on heating,.
That is explained like this:
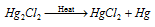
It gets dissolved in chlorine water creating mercuric chloride.
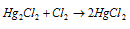
It turns black in color because of the formation of a mixture of finely divided black Hg and mercuric amino chloride, along with the ammonia.
Explained as follow:
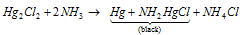
It is used to make standard calomel electrode and like the purgative in medicine.
(E) Mercuric sulphide, HgS : The solubility product of HgS is lower as compared that of ZnS and therefore it gets precipitated since black solid while H2S is passed by an acidic solution of any mercury (II) salt.
Equation:
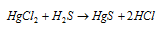
It is not soluble in water and HCl but dissolves in aqua regia (1 part conc. HNO3 and 3 parts conc. HCl)

The color varies to red and therefore it is used like a red pigment, on the sublimation of it.
(F) Mercuric sulphate, HgSO4 : It is acquired while HgS is treated along with conc.H2SO4 (Sulfuric acid).
Explained as follow:
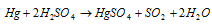
This is a white solid which decomposes on heating to provide mercurous sulphate.
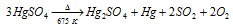
It is employed like a catalyst in the hydration of alkynes to provide aldehydes or ketones. It can as well be used like a cosmetic in the name Vermillon and in ayurvedic medicine like makardhwaj.
(G) Amalgams: Mercury makes alloys that are generally known as amalgams, with all metals apart from iron and platinum. Hence it is transported in iron containers.
(H) Alloy of transition metal: it is described in the table that is discussed before in metallurgy.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Compounds of Mercury questions? Compounds of Mercury topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Compounds of Mercury related problems. We provide step by step Compounds of Mercury question's answers with 100% plagiarism free content. We prepare quality content and notes for Compounds of Mercury topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours