The compounds of gold
(1) Auric chloride, AuCl3 : It can prepared by passing dry Cl2 over finely divided gold powder at the temperature of 573 K
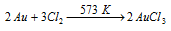
This is a red colored crystalline solid which is soluble in water and decomposes on heating to give gold (I) chloride and Cl2
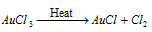
It dissolves in concentratedHCl forming chloroauric acid
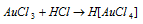
Chloroauric acid is used in the photography for toning silver prints and as an antidote for the snake poisoning.
(2) Aurous sulphide, Au2S : It is prepared when H2S is passed through an acidified solution of potassium aurocyanide, K[Au(CN)2]
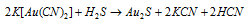
It is a dark brown solid, not attached by the dilute mineral acids and therefore is probably the most stable gold compound.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Compounds of Gold questions? Compounds of Gold topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Compounds of Gold related problems. We provide step by step Compounds of Gold question's answers with 100% plagiarism free content. We prepare quality content and notes for Compounds of Gold topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours