Compounds of Copper:
(a) Halides of copper: Copper chloride which is denoted by CuCl2 is prepared through passing chlorine over heated copper. The solution of CuCl2 is dark brown but after dilution it will become change first to green and after that blue color.
When it communicate with heat, it disproportionate to copper chloride and chlorine
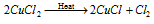
The above given equation is used as a catalyst in the Daecon's procedure for manufacture the chlorine.
In water copper chloride (CuCl) is a white insoluble. By boiling a solution of CuCl2 copper chloride can be attained along with excess of concentrated HCl and copper turnings the equation for that is given below:
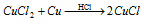
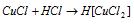
It dissolves in conc. HCl due to the creation of complex H [CuCl2] that was show in the above equation
Additionally it is taken in use as catalyst along with NH4Cl by the preparation of synthetic rubber.
(b) Cuprous oxide Cu2O: Cuprous Oxide that is also known as copper oxide is a reddish brown powder insoluble in water and used to inform red color to glass in glass industry but soluble in ammonia solution, while it create diammine copper (I) ion that was denoted as 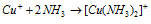 .
.
(c) Cupric oxide CuO : Cupric oxide which is denoted by CuO have dark black color and hygroscopic powder that is reduced to Cu through the hydrogen, CO and etc. By heating the copper nitrate it is prepared. On the glass cupric oxide pass light blue color.
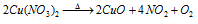
(d) Copper sulphate CuSO4.5H2O (Blue vitriol): while dilute H2SO4 on copper scrap coming in action in presence of air it is prepared. The equation of Copper Sulphate is given below
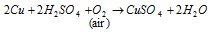 ... (1)
... (1)
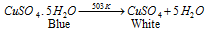 ... (2)
... (2)
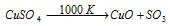 ... (3)
... (3)
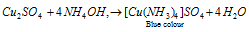 ... (4)
... (4)
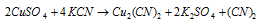 ... (5)
... (5)
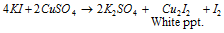 ... (6)
... (6)
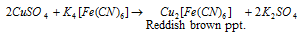
Equation (1): on heating this blue salt becomes white because of loss of water of crystallization.
Equation (2): at about 1000 K, CuSO4 decomposes to provide CuO and SO3
Equation (3): It gives a deep blue solution of tetrammine copper (II) sulphate along with NH4OH
Equation (4): It provides yellow precipitate first of CuCN with the KCN that decomposes of give Cu2(CN)2. It dissolves in excess of KCN to give K3[Cu(CN)4]
Equation (5): It gives white ppt. with KI of Cu2I2
Equation (6): CuSO4 provides a reddish brown precipitate of Cu2[Fe(CN)6] with K4[Fe(CN)6]
Uses: It is used in making Bordeaux mixture of 11 elements lime as milk of lime + 16 elements copper sulphate in 1,000 parts of water and also used as a mordant within dyeing for electroplating and electrorefining of copper. For formed green pigments holding copper carbonate and other compounds of copper it is a fine fungicide. the fungicide is used in starch paste for book binding work. Like as Verdigris that is Cu(CH3COO)2Cu(OH)2 which is basic copper acetate and is used as a green pigment in paints.
(e) Cupric sulphide CuS : Cupric sulphide which is denoted by CuS can be prepared as given below:
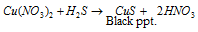
(f) Basic copper carbonates: The normal carbonate does not exist because of lower solubility of the hydroxide. The malachite CuCO3, Cu(OH)2 are the two primary copper carbonates occur in nature which has a fine azurite and green colour, 2CuCO3.Cu(OH)2 expression represent the deep blue in colour.
By heating the mixture of CuSO4 solution the malachite can prepare and in a sealed tube of limestone at 423 - 443 K. the given equation represent it:

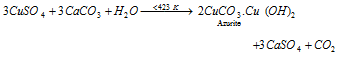
On the lower temperature azurite is create the above equation
While heating it, both are decompose to provide water, black cupric oxide, andCO2.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Compounds of Copper questions? Compounds of Copper topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Compounds of Copper related problems. We provide step by step Compounds of Copper question's answers with 100% plagiarism free content. We prepare quality content and notes for Compounds of Copper topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours