Compounds of Aluminium
(i) Aluminium oxide or Alumina (Al2O3) : It occurs in nature as colourless corundum and several coloured minerals like ruby (red), topaz (yellow), Sapphire (blue), amethyst (violet) and emerald (green). These minerals are taken in use as precious stones (gems).
(ii) Aluminium chloride (Al2Cl6) : It is prepared by passing dry chlorine over aluminium powder.
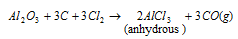
It exists as dimer Al2Cl6, in inert organic solvents and in vapour state. It sublimes at 100 0C under vacuum. Dimeric structure disappears when AlCl3 is dissolved in water. This is hygroscopic in character and absorbs moisture when exposed to air.
(iii) Thermite : A mixture of aluminium powder and Fe2O3 in the ratio 1:3. It is used in the welding of iron. The reaction between Al and Fe2O3 is highly exothermic,
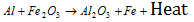
(iv) Aluminium sulphate [Al2(SO4)3] : It is used for the preparation of alums e.g., potash alum Al2(SO4)3.K2SO4.24H2O. It is also used for making fire proof clothes.
(iv) Alums : In general, the term alum is given to double sulphates of the type M2SO4.M'2(SO4)3.24H2O where M is a univalent cation like Na+, K+ and NH4+,M' is a trivalent cation like Al3+, Fe3+ and Cr3+.
Some significant points to be noted about the alums are
(a) General formula is M2SO4.M'2(SO4)3.24H2O
M = Monovalent metal; M' =Trivalent metal
In an alum crystals, 6 water molecules are held by the monovalent ion, 6 water molecules are held by the trivalent ion, 12 water molecules are held in crystal structure.
(b) All the alums are isomorphous. Aqueous solutions of alums are acidic because of cationic hydrolysis of trivalent cation.
(c) Double sulphates of the divalent ions and trivalent ions with 24 water molecules in their crystals are known as Pseudoalums. Common formula is MSO4.M'2(SO4)3.24H2O
M= Bivalent metal; M'= Trivalent metal
(d) The pseudoalums are not isomorphous with alums.
(e) Feather alum or the Hair-salt which can be given as Al2SO4.18H2O is native form of the aluminium sulphate.
(f) Potash alum is taken in use for tanning of leather, as mordant in the dyeing and calico printing, for sizing paper, as a syptic to stop bleeding and the purification of water.
Some essential alums are
Potash alum K2SO4.Al2(SO4)3.24H2O
Sodium alum Na2SO4.Al2(SO4)3.24H2O
Ammonium alum (NH4)2SO4.Al2(SO4)3.24H2O
Chrome alum K2SO4.Cr2(SO4)3.24H2O
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Compounds of Aluminium questions? Compounds of Aluminium topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Compounds of Aluminium related problems. We provide step by step Compounds of Aluminium question's answers with 100% plagiarism free content. We prepare quality content and notes for Compounds of Aluminium topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours