Reaction
|
Acetaldehyde
|
Acetone
|
Similarities
|
|
|
|
1. Reduction with H2 and Ni or LiAlH4
|
Forms ethyl alcohol

|
Forms isopropyl alcohol

|
|
2. Clemmensen's reduction
(Zn/Hg and conc. HCl)
|
Forms ethane (an alkane)

|
Forms propane (an alkane)

|
|
3. Addition of HCN
|
Forms acetaldehyde cyanohydrin
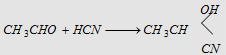
|
Forms acetone cyanohydrin
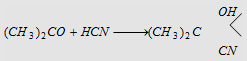
|
|
4. Addition of NaHSO3
|
White crystalline derivative
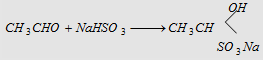
|
White crystalline derivative
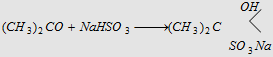
|
|
5. Grignard reagent followed by hydrolysis
|
Forms isopropyl alcohol
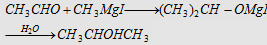
|
Forms tertiary butyl alcohol
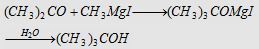
|
|
6. With hydroxylamine
(NH2OH)
|
Forms acetaldoxime (an oxime)
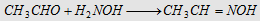
|
Forms acetoxime (an oxime)

|
|
7. With hydrazine (NH2NH2)
|
Forms acetaldehyde hydrazone

|
Forms acetone hydrazone
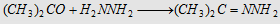
|
|
8. With phenyl hydrazine (C6H5NHNH2)
|
Forms acetaldehyde phenylhydrazone
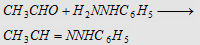
|
Forms acetone phenyl hydrazone
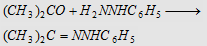
|
|
9. With semicarbazide
(H2NNHCONH2)
|
Forms acetaldehyde semicarbazone
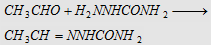
|
Forms acetone semicarbazone
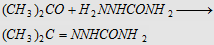
|
|
10. With PCl5
|
Forms ethylidene chloride (Gem dihalide)
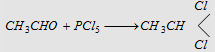
|
Forms isopropylidene chloride (Gem dihalide)
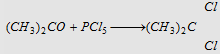
|
|
11. With chlorine
|
Forms chloral (Gem trihalide)
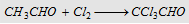
|
Forms trichloro acetone (Gem trihalide)
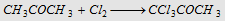
|
|
12. With alcohols
|
Forms acetal (a diether)
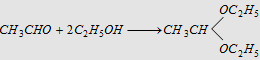
|
Forms ketal (a diether)
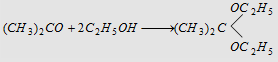
|
|
13. With SeO2
|
Forms glyoxal

|
Forms methyl glyoxal
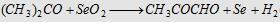
|
|
14. Iodoform reaction
(I2 + NaOH)
|
Forms iodoform
|
Forms iodoform
|
|
15. Bleaching powder
|
Forms chloroform
|
Forms chloroform
|
|
16. Aldol condensation with mild alkali
|
Forms aldol
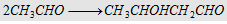
|
Forms diacetone alcohol
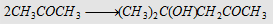
|
|
17. Polymerisation
|
Undergoes polymerisation
|
Does not undergo polymerisation but gives condensation reaction
|
|
18. With NH3
|
Forms acetaldehyde ammonia
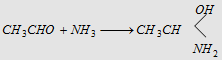
|
Forms diacetone ammonia
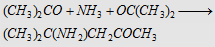
|
|
19. With conc. NaOH
|
Forms brownish resinous mass
|
No reaction
|
|
20. With HNO2
|
No reaction
|
Forms oximino acetone

|
|
 21. With chloroform 21. With chloroform
|
No reaction
|
Forms chloretone
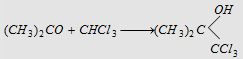
|
|
22. With alk. sodium nitroprusside
|
Deep red colour
|
Red colour changes to yellow on standing
|
|
23. With sodium nitroprusside + Pyridine
|
Blue colour
|
No effect
|
|
24. Boiling point
|
21o C
|
56o C
|
|
Dissimilarities
|
|
|
|
25. With Schiff's reagent
|
Pink colour
|
Does not give pink colour
|
|
26. With Fehling's solution
|
Gives red precipitate
|
No reaction
|
|
27. With Tollen's reagent
|
Gives silver mirror
|
No reaction
|
|
28. Oxidation with acidified
K2Cr2O7
|
Easily oxidised to acetic acid

|
Oxidation occurs with difficulty to form acetic acid

|