Some of the Commercial cell (Batteries)
One of the major usage of the galvanic cells is the generation of portable electrical energy. These types of cells are also popularly known as batteries. The term battery is usually used for two or more Galvanic cells connected in series. Therefore, a battery is an arrangement of electrochemical cells used as an energy source. Basis of the electrochemical cell is an oxidation - reduction reaction.
Types of commercial cells: There are majorly two types of commercial cells,
(1) Primary cells : In these type of cells, the electrode reactions cannot be reversed by an external electric energy source. In these type of cells, reactions occur only once and after use they become dead. Thus, they are not chargeable. Some general example are, mercury cell, dry cell, Daniell cell and alkaline dry cell
(i) Voltaic cell
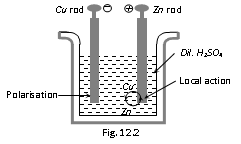
Cathode is Cu rod Anode is Zn rod
Electrolyte : dil. H2SO4 Emf : 1.08 V
At cathode : 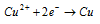
At Anode : 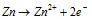
Over all reaction : 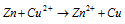
(ii) Daniel cell
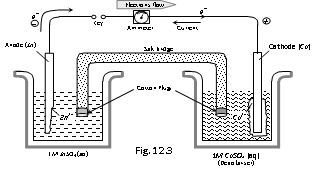
Cathode : Cu rod Anode : Zn rod
Electrolyte : dil. H2SO4 Emf : 1.1 V
At cathode : 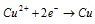
At Anode : 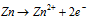
Over all reaction : 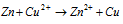
(iii) Lechlanche cell (Dry cell)
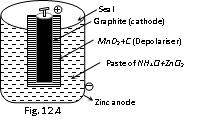
Cathode : Graphite rod Anode : Zn pot
Electrolyte : Paste of NH4Cl + ZnCl2 in starch
Emf : 1.2 V to 1.5 V
At cathode : 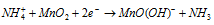
At Anode : 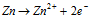
Over all reaction : 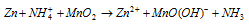
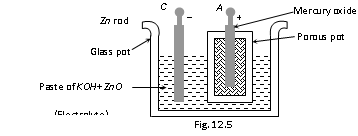
(iv) Mercury cell
Cathode : Mercury (II) oxide Anode : Zn rod
Electrolyte : Paste of KOH + ZnO Emf : 1.35 V
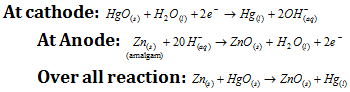
(2) Secondary cells : In the secondary cells, reactions can be reversed by the external electrical energy source. Thus, these cells can be recharged by passing electric current and used again and again. These are also known as storage cells. The examples of secondary cells are, the lead storage battery and nickel cadmium storage cell.
|
In charged
|
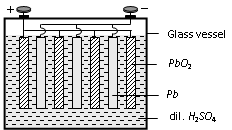
Lead storage cell
|
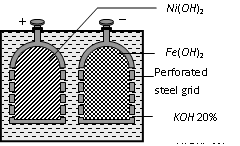
Alkali cell
|
|
|
|
|
|
Positive electrode
|
Perforated lead plates coated with PbO2
|
Perforated steel plate coated with Ni(OH)4
|
|
Negative electrode
|
Perforated lead plates coated with pure lead
|
Perforated steel plate coated with Fe
|
|
Electrolyte
|
dil. H2SO4
|
20% solution of KOH + 1% LiOH
|
|
During charging
|
Chemical reaction
At anode : PbSO4 + 2H+ + 2e- → Pb + H2SO4
At cathode : PbSO4 + SO4- - + 2H2O - 2e- → PbO2
+ 2H2SO4
Specific gravity of H2SO4 increases and when specific gravity becomes 1.25 the cell is fully charged.
Emf of cell: When cell is fully charged then E = 2.2 volt
|
Chemical reaction
At anode : Ni (OH)2 + 2OH+ - 2e- → Ni(OH)4
At cathode : Fe(OH)2 + 2K+ + 2e- → Fe + 2KOH
Emf of cell : When cell is fully charged then E = 1.36 volt
|
|
During discharging
|
Chemical reaction
At anode : Pb + SO4- - - 2e- → PbSO4
At cathode : PbO2 + 2H+ + 2e- + H2SO4 → PbSO4 +
2H2O
Specific gravity of H2SO4 decreases and when specific gravity falls below 1.18 the cell requires recharging.
Emf of cell : When emf of cell falls below 1.9 volt the cell requires recharging.
|
Chemical reaction
At anode : Fe + 2OH- - 2e- → Fe(OH)2
At cathode : Ni(OH)4 + 2K+ + 2e- → Ni(OH)2 +
2KOH
Emf of cell : When emf of cell falls below 1.1 V it requires charging.
|
|
Efficiency
|
80%
|
60%
|
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Commercial cell (Batteries) questions? Commercial cell (Batteries) topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Commercial cell (Batteries) related problems. We provide step by step Commercial cell (Batteries) question's answers with 100% plagiarism free content. We prepare quality content and notes for Commercial cell (Batteries) topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours