The Close packing in three dimensions: In order to expand three the dimensional close packing, let us retain hexagonal close packing in the first layer. For the close packing, each spheres in second layer rests in the hollow at the centre of the three touching spheres in the layer as shown in figure drawn below. The spheres in first layer are shown by the solid lines while those present in the second layer are shown by the broken lines. It can be noted that only half voids of the triangular in the first layer are occupied by the spheres in the second layer (that is either b or c). The unoccupied hollows or the voids in the first layer are signified by (c) in figure drawn below.
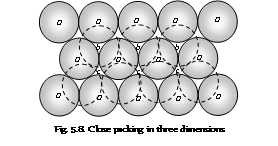
There are two alternative methods in which the species in third layer can be arranged above the second layer,
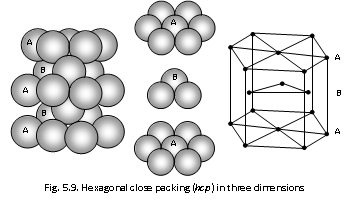
(i) The Hexagonal close packing : Third layer lies vertically over the first and the spheres in the third layer rest in one pattern of the hollows on the top of second layer. This pattern is called as ABAB .... type and 74% of available space is occupied by the spheres. This type of arrangement is found in the components such as Be, Ti, Zn, Cd, Sc, Y, Tc, Zr, Mg, Ru.
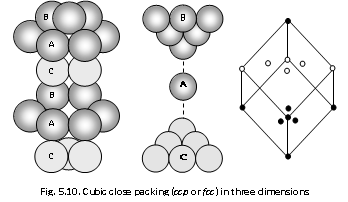
(ii) The Cubic close packing : The third layer is dissimilar from first and the spheres in the third layer lie on the other set of the hollows marked 'C' in first layer. This arrangement is termed as ABCABC..... type and in this also 74% of the available space is occupied by the spheres only. The cubic close packing has the face centred cubic (fcc) unit cell. This type of arrangement is seen in Cu, Ag, Au, Ni, Pt, Pd, Co, Rh, Ca, Sr.
(iii) The Body centred cubic : This arrangement of the spheres (or atoms) is not exactly close packed. This structure can be achieved if spheres in the first layer (A) of the close packing are a bit opened up. It results as none of these spheres are in the contact with each other. Second layer of spheres (B) can be placed on the top of first layer such that each sphere of the second layer is in touch with the four spheres of the layer below it. Successive building of third will be exactly like the first layer. This pattern of building the layers is repeated number of times we get an arrangement as shown in the figure drawn below. This arrangement is found in the atoms such as Li, , Cs, Mo, Na, K, Ba, Rb, V, Cr, Nb, Fe.
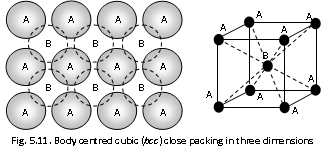
Table : 5.3 Comparison of hcp, ccp and bcc
|
Property
|
hcp
|
ccp
|
bcc
|
|
Arrangement of packing
|
Close packed
|
Close packed
|
Not close packed
|
|
Type of packing
|
AB AB A...
|
ABC ABC A...
|
AB AB A...
|
|
Available space occupied
|
74%
|
74%
|
68%
|
|
Coordination number
|
12
|
12
|
8
|
|
Malleability and ductility
|
Less malleable, hard, brittle
|
Malleable and ductile
|
|
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Close packing in three dimensions questions? Close packing in three dimensions topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Close packing in three dimensions related problems. We provide step by step Close packing in three dimensions question's answers with 100% plagiarism free content. We prepare quality content and notes for Close packing in three dimensions topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours