Chemical Properties
(i) Basic nature (Salt formation): It behaves as a weak monoacid base (Kb = 1.5 * 10-14). It forms solt with strong acid.
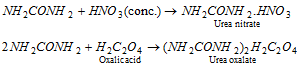
Urea is a stronger base than ordinary amide. It is because of the resonance stabilization of cation, the negatively charged oxygen atom is capable of coordination with one proton.
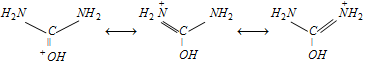
q An aqueous solvent of urea is neutral.
(ii) Hydrolysis
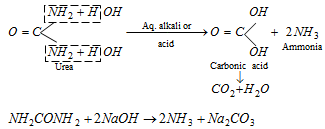
An enzyme, urease, present in soyabean and soil also brings hydrolysis .
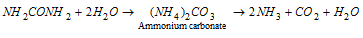
(iii) Action of heat
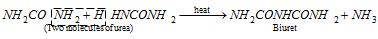
Urea is identified by the test known as biuret test. The biuret residue is dissolved in water and made alkaline with a few drops of NaOH. When a part of copper sulphate solution is added to the alkaline solution of biuret, a violet colouration is produced.
when heated rapidly at 170o C, polymerisation takes place:
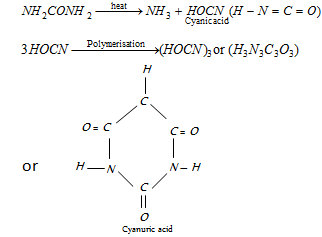
(iv) Reaction with nitrous acid
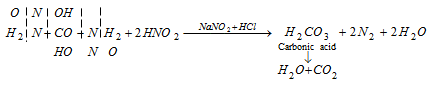
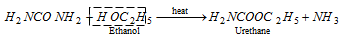
(v) Reaction with ethanol
(vi) Reaction with chlorine water
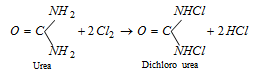
(vii) Dehydration
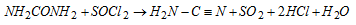
(viii) Reaction with fuming sulphuric acid
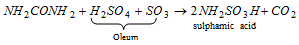
(ix) Formation of cyclic ureides
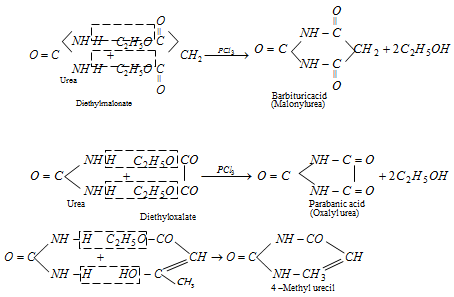
(x) Reaction with formaldehyde
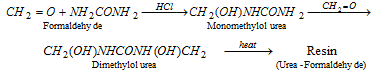
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Chemical Properties of Urea questions? Chemical Properties of Urea topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Chemical Properties of Urea related problems. We provide step by step Chemical Properties of Urea question's answers with 100% plagiarism free content. We prepare quality content and notes for Chemical Properties of Urea topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours