Chemical properties
(i) Action of heat: Potassium dichromate when heated strongly. This decomposes to release oxygen.
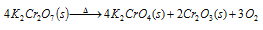
(ii) Action of acids
(a) In the cold, with concentrated H2SO4, red crystals of the chromium trioxide separate out.

On heating the dichromate-sulphuric acid mixture, oxygen gas is released.
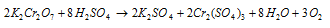
(b) With the HCl, on heating chromic chloride is formed and Cl2 is released.
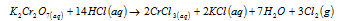
(iii) Action of alkalies : With alkalies, it gives result to chromates. Hear its shown with KOH,
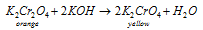
On acidifying, the colour again changes to orange-red owing to the formation of dichromate.
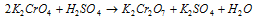
In fact, in dichromate solution, the Cr2O72- ions are in equilibrium with CrO42- ions.
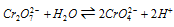
(iv) Oxidising nature : In neutral or in acidic solution, the potassium dichromate acts as an outstanding oxidising agent, and Cr2O72- gets reduced to Cr3+. The standard electrode potential for the reaction,
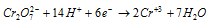 is +1.31V.
is +1.31V.
This indicates that dichromate ion is a fairly strong oxidising agent, especially in strongly acidic solutions. This is the reason why potassium dichromate is widely used as an oxidising agent, for quantitative estimation of the reducing agents such as, Fe2+. It oxidises,
(a) Ferrous salts to ferric salts
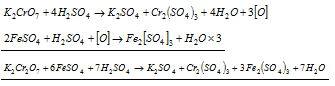
Ionic equation
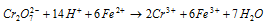
(b) The sulphites to sulphates and arsenites to arsenates.
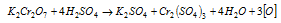

Ionic equation
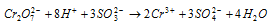
Likewise, arsenites are oxidised to arsenates.
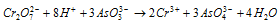
(c) Hydrogen halides to halogens.

where, X may be Cl, Br, I.
Ionic equation :
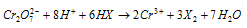
(d) Iodides to iodine
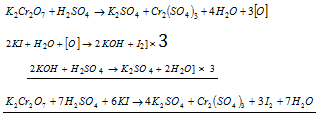
Ionic equation : 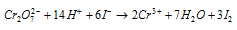
Therefore, when KI is added to an acidified solution of K2Cr2O7 iodine gets liberated.
(e) It oxidises H2S to S.
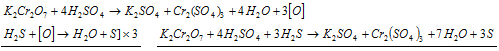
Ionic equation
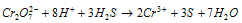
(v) Formation of insoluble chromates : With soluble salts of lead, barium and more, the potassium dichromate gives insoluble chromates. Lead chromate is the significant yellow pigment.

(vi) Chromyl chloride test : When potassium dichromate is heated with conc. H2SO4 in the presence of a soluble chloride salt, the orange-red vapours of chromyl chloride (CrO2Cl2) are formed.
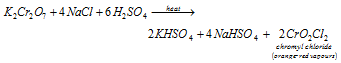
Chromyl chloride vapours when passed through water give yellow-coloured solution containing chromic acid.
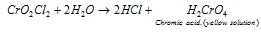
The chromyl chloride test can be used for detection of chloride ion is any mixture.
Uses : Potassium dichromate is used as,
(i) An oxidising agent
(ii) In chrome tanning
(iii) The basic raw meterial for the preparing large number of chromium compounds
(iv) The primary standard in volumetric analysis.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Chemical Properties of Potassium Dichromate questions? Chemical Properties of Potassium Dichromate topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Chemical Properties of Potassium Dichromate related problems. We provide step by step Chemical Properties of Potassium Dichromate question's answers with 100% plagiarism free content. We prepare quality content and notes for Chemical Properties of Potassium Dichromate topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours