Chemical properties of Phenol
(i) Acidic nature : Phenol is a weak acid. The acidic nature of phenol is due to the formation of stable phenoxide ion in solution.
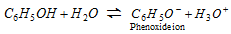
The phenoxide ion is stable due to resonance.
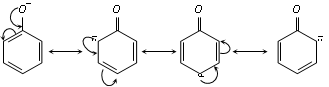
The negative charge is spread throughout the benzene ring. This charge delocalisation is a stabilising factor in the phenoxide ion and increase acidity of phenol. [No resonance is possible in alkoxide ions (RO-) derived from alcohols. The negative charge is localised on oxygen atom. Thus alcohols are not acidic].
q Phenols are much more acidic than alcohols but less so than carboxylic acids or even carbonic acid. This is shown by the values of ionisation constants. The relative acidity follows the following order
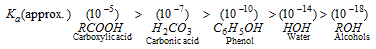
Effects of substituents on the acidity of phenols : Presence of electron attracting group, (e.g., -NO2, -X, -NR3+, -CN, -CHO, -COOH)on the benzene ring increases the acidity of phenol as it enables the ring to draw more electrons from the phenoxy oxygen and thus releasing easily the proton. Further, the particular effect is more when the substituent is present on o- or p-position than in m-position to the phenolic group.
The relative strengths of some phenols (as acids) are as follows :
p-Nitrophenol > o-Nitrophenol > m- Nitrophenol > Phenol
Presence of electron releasing group, (e.g., -CH3, -C2H5, -OCH3, -NR2) on the benzene ring decreases the acidity of phenol as it strengthens the negative charge on phenoxy oxygen and thus proton release becomes more difficult. Thus, cresols are less acidic than phenol.
However, m-methoxy and m-aminophenols are stronger acids than phenol because of -I effect and absence of +R effect.
m-methoxy phenol > m-amino phenol > phenol > o-methoxy phenol > p-methoxy phenol
Chloro phenols : o- > m- > p-
Cresols : m- > p- > o-
Dihydric phenol : m- > p- > o-
The acidic nature of phenol is observed in the following :
(a) Phenol changes blue litmus to red.
(b) Highly electropositive metals react with phenol. 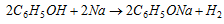
(c) Phenol reacts with strong alkalies to form phenoxides. 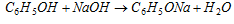
However, phenol does not decompose sodium carbonate or sodium bicarbonate, i.e., CO2 is not evolved because phenol is weaker than carbonic acid.
(ii) Reactions of -OH group
(a) Reaction with FeCl3 : Phenol gives violet colouration with ferric chloride solution (neutral) due to the formation of a coloured iron complex, which is a characteristic to the existence of keto-enol tautomerism in phenols (predominantly enolic form).
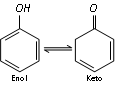
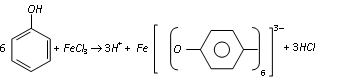
This is the test of phenol.
(b) Ether formation : Phenol reacts with alkyl halides in alkali solution to form phenyl ethers (Williamson's synthesis). The phenoxide atom is a nucleophile and can replace halogenation of alkyl halide.
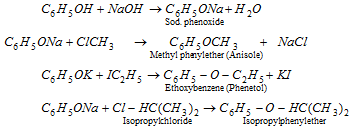
Ethers are also generated when vapours of phenol and an alcohol are heated over thoria (ThO2) or Al2O3.
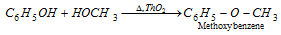
(iii) Reactions of benzene nucleus : The -OH group is ortho and para directing. It activates the benzene nucleus.
(a) Halogenation : Phenol reacts with bromine in carbon disulphide (or CHCl3) at low temperature to form mixture of ortho and para bromophenol.
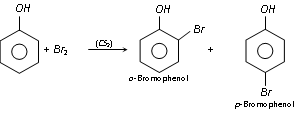
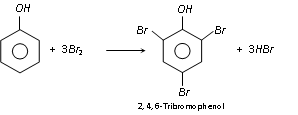
Phenol generates a white precipitate with excess of bromine water yielding 2, 4, 6-tribromophenol.
(b) Sulphonation : Phenol reacts with conc. H2SO4 readily to form mixture of ortho and para hydroxy benzene sulphonic acids.
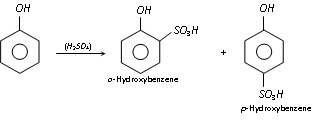
At low temperature (25°C), the ortho-isomer is the major product, whereas at 100°C, it gives mainly the para-isomer.
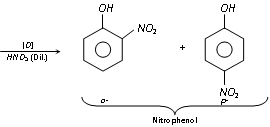
(c) Nitration : Phenol reacts with dilute nitric acid at 5-10°C to form ortho and para nitro phenols, but the yield is meager because of oxidation of phenolic group set. The -OH group is reactivating set, hence nitration is possible with light nitric acid.
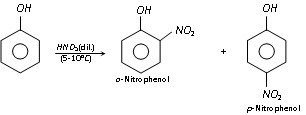
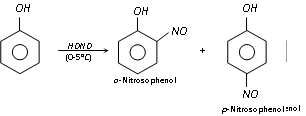
It is supposed that the mechanism of the above reaction involves the formation of o- and p- nitroso phenol with nitrous acid, HNO2 (NaNO2 + HCl) at 0-5°C, which gets oxidised to o- and p- nitrophenol with dilute nitric acid.
However, when phenol is treated with concentrated HNO3 in presence of concentrated H2SO4, 2,4,6-trinitrophenol (Picric acid) is formed.
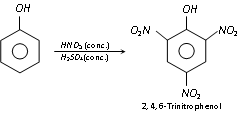
To get better yield of picric acid, first sulphonation of phenol is prepared and then nitrated. Presence of  group prevents oxidation of phenol.
group prevents oxidation of phenol.
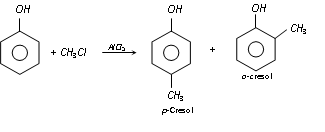
(d) Friedel-Craft's reaction : Phenol when treated with methyl chloride in presence of anhydrous aluminium chloride, p-cresol is the main substances. A very short amount of o-cresol is also formed.
RX and AlCl3 give poor yields because AlCl3 coordinates with O. So Ring alkylation takes place as follows,
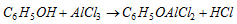
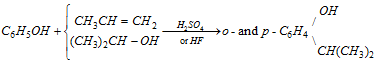
Thus to carry out successful Friedel-Craft's reaction with phenol it is necessary to use a large amount of AlCl3. The Ring alkylation takes place as follows :
(e) Reimer-Tiemann reaction :
Phenol, on refluxing with chloroform and sodium hydroxide (aq.) given by acid hydrolys
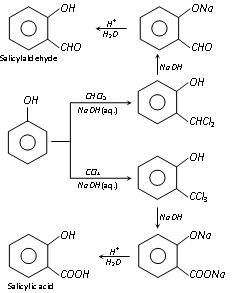
(o-hydroxy benzaldehyde) and a very small amount of p-hydroxy benzaldehyde. However, when carbon tetrachloride is needed, salicylic acid (predominating product) is formed.
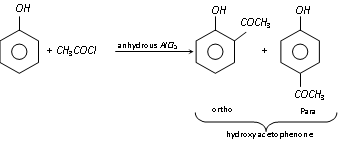
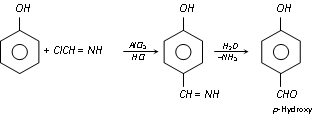
(f) Gattermann's reaction : Phenol, when treated with liquid hydrogen cyanide and hydrochloric acid gas in presence of anhydrous aluminium chloride yields mainly p-hydroxy benzaldehyde (Formylation).
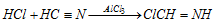
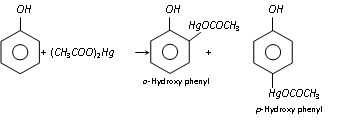
(g) Mercuration
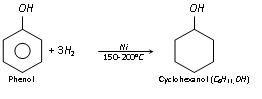
(i) Hydrogenation
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Chemical properties of Phenol questions? Chemical properties of Phenol topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Chemical properties of Phenol related problems. We provide step by step Chemical properties of Phenol question's answers with 100% plagiarism free content. We prepare quality content and notes for Chemical properties of Phenol topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours