Chemical properties
(1) Hydrides
(i) Elements of group 13 do not react directly with hydrogen but a number of polymeric hydrides are known to exist.
(ii) Boron forms a large no. of volatile covalent hydrides, known as boranes e.g. B2H6, B4H10, B5H11, B6H10 Two series of boranes with general formula BnHn+4 and BnHn+6 are more important.
(iii) Boranes are electron deficient compounds. It is important to note that although BX3 are well known, BH3 is not known. This is due of the fact that hydrogen atoms in BH3 have no free electrons to form pp-pp back bonding and thus boron has incomplete octet and hence BH3 molecules dimerise to form B6H6 having covalent and three centre bonds.
(iv) Al forms only one polymeric hydride (AlH3)n commonly known as alane It contains Al---H----Al bridges.
(v) Al and Ga forms anionic hydrides e.g. LiAlH4 and LiGa H4,
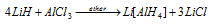
(2) Reactivity towards air
(i) Pure boron is almost unreactive at ordinary temperature. It reacts with air to form B2O3 when heated it does react with water. Al burns in air with evolution of heat give Al2O3.
(ii) Ga and In are not affected by air even when heated whereas Tl is little more reactive and also form an oxide film at surface. In moist air, a layer of Tl (OH) is formed.
(iii) Al decomposes H2O and reacts readily in air at ordinary temperature to form a protective film of its oxides which protects it from further action.
(3) Oxides and hydroxides
(i) The members of the boron family form oxide and hydroxides of usual formula M2O3 and M (OH)3 respectively.
(ii) The acidic nature of oxides and hydroxides changes from acidic to basic through amphoteric from B to Tl.
B2O3 and B(OH)3> Al2O3 and Al(OH)3 >
(acidic in nature) (amphoteric in nature)
Ga2O3 and Ga(OH)3> In2O3 In (OH)3> Tl2O3 Tl(OH)3
(amphoteric) (basic) (strong basic)
B(OH)3 or H3BO3 is weak monobasic Lewis acid.
(iii) Boric acid, B(OH)3 is although soluble in water as it accepts lone pair of the electron to act as Lewis acid. Remaning all hydroxides of group 13 are insoluble in water and form the gelatinous precipitate.
B(OH)3 + H2O →B(OH)41-+H+
(iv) Al2O3 being amphoteric in nature dissolves in acid and alkalies both.
Al2O3 + 3H2SO4→ Al2 (SO4)3 + 3H2O
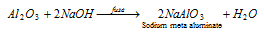
(v) One of the crystalline forms of alumina (Al2O3) is known as corrundum. It is quite hard and used as abrasive. It is prepared by heating the amorphous form of Al2O3 to 2000 K.
(4) Action of Acids
(i) Boron does not react with non oxidizing acids; though, it dissolves in the nitric acid to form boric acids.
(ii) Ga Al, and In dissolve in acids forming their trivalent cations; though, Al and Ga become passive because of the formation of the protective film of oxides.
(iii) Thallium gets dissolved in acids forming univalent cation and becomes passive in HCl because of the formation of water insoluble TICl.
(5) Action of Alkalies
(i) Boron dissolves only in the fused alkalies,
2B + 6NaOH (fused)→ 2Na3BO3 + 3H2
(ii) Al and Ga dissolves in the fused as well as in aqueous alkalies, 2Al + 2 NaOH + 2H2O →2NaAl O2 + 3H2
(iii) Indium does not get affected in alkalies even on heating and remains same.
(6) Halides
(i) All the group 13 elements from trihalides, MX3 on directly combining with the halogens.
M + X2 → MX3
(ii) All the trihalides of group 13 elements are known except Tl (III) iodide.
(iii) Because of small size and high electronegativity of the boron, all boron halides are covalent and Lewis acids. These exist as monomeric molecules possesing plane triangular geometry (sp2 hybridization).
(iv) All the Boron trihalides except the BF3 are hydrolysed to boric acid.
BX3+ 3H2O →B(OH)3 + 3HX; [X=Cl, Br, I]
However, BF3 forms as addition product with water,

BF3 showing less tendency for hydrolysis as well as Lewis acid nature, is broadly used as a catalyst in organic reactions for example Friedel- Crafts reaction.
(v) Boron atom, in BX3, contains six electrons in the outermost orbit and thus it can accept a pair of electrons forms a donor molecule like NH3 to complete its octet. Hence boron halides act as very efficient Lewis acids. The relative Lewis acid character of the boron trihalides is found to obey the order ; BI3>BBr3>BCl3>BF3.
Though, the above order is just the reverse of normally expected order on basis relative electronegativities of the halogens. Fluorine, is the most electronegative, must create the greatest electron deficiency on the boron and hence B in BF3 should accept electron pair from the donor quite rapidly than in other boron trihalides. However this is not true.
This anomalous behaviour has been explained on the basis of the relative tendency of halogen atom to back-donate its unutilised electrons to the vacant p orbitals of boron atom. In the boron trifluoride, each of the fluorine has completely filled unutilised 2p orbitals while boron has a vacant 2p orbital. Now as both of these orbitals belong to same energy level (2p) they can overlap the effectively as a result of fluorine electrons are transferred into the vacant 2p orbital of boron resulting in formation of an additional pp-pp bond. This type of bond formation is called as back bonding or back donation. Therefore the B- F bond has some double bond character. Back bonding might take place between boron and of the three fluorine atoms and thus boron trifluoride is regarded as a resonance hybrid of some structures.
Resonance in boron trifluoride is also evidenced by the fact that the three boron-fluorine bonds are identical and are shorter than the usual single boron-fluorine bond As a result of back bonding, electron deficiency of boron is reduced and hence Lewis acid nature is decreased. The affinity for the formation of back bonding (pp- pp bond) is maximum in BF3 and decreases very rapidly from BF3 to BI3 This is probably because of the fact that overlapping of the vacant 2p orbitals of boron cannot take place simply with the p-orbitals of high energy levels (3p in Cl, 4p in Br and 5p in iodine). Therefore BI3Br3 and BCl3 are stronger Lewis acids than the BF3.
(vi) Lewis acid character of the halides of group 13 elements decreases in order, B > Al > Ga > In.
(vii) Boron halides form complex halides of the type, [BF4-], in which boron atom extends its coordination number to four by utilising empty p-orbital. Its coordination number cannot be extended beyond four due to non availability of d-orbitals. Though, the other trihalides of this group form complex halides of the type (AlF6)3-, (GaCl6)3- and (InCl6)3-, etc where the central atom extends its coordination number to 6 by the use of d-orbitals.
(viii) The fluorides of Al, Ga In and Tl are ionic and have high melting points. The high melting points of the metal fluorides can be explained on the basis that their cations are sufficiently large and have vacant d-orbitals for attaining a coordination number of six towards the relatively small fluorine atom.
(ix) Other halides of Al, Ga, In and Tl are largely covalent in anhydrous state and possess low melting point. The halides do not show the backbonding because of increases in the size of the element. Though, make use of the vacant p-orbitals by co-ordinate bond that is metal atoms complete their octet by forming dimers. Therefore aluminium bromide, aluminium chloride, and indium iodide exist as dimers, in the vapour state and in non-polar solvents both.
The dimer structure of the Al2Cl6 is evidenced by the below stated facts,
(a) Vapour density of aluminium chloride measured at 4000C corresponds to formula Al2Cl6.
(b) Bond distance among aluminium chlorine bond forming bridge is greater (2.21Å) than distance between aluminum-chlorine bond present in the end (2.06 Å). The dimeric structure disappears when the halides are dissolved in water This is due to high heat of hydration which split the dimeric structure into [M(H2O)6]3+ and 3X- ions and the solution becomes good conductor of electricity.
Al2Cl6 + 2H2O → 2[Al(H2O)6]3++6Cl- ; Therefore Al2Cl6 is ionic in water.
The dimeric structure may also split by reaction with donor molecules for example R3N. This is because of the formation of complexes of the type R3NAlCl3 The dimeric structure of Al2Cl6 exist in vapour state below 473K and at higher temperature it dissociates to trigonal planar AlCl3 molecule.
Boron halides do not exist as dimer because of small size of boron atom which makes it unable to co-ordinate the four large-sized halide ions.
(x) BF3 and AlCl3 acts as catalyst and Lewis acid in several of the industrial process.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Chemical Properties of Boron Family questions? Chemical Properties of Boron Family topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Chemical Properties of Boron Family related problems. We provide step by step Chemical Properties of Boron Family question's answers with 100% plagiarism free content. We prepare quality content and notes for Chemical Properties of Boron Family topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours