Chemical properties
(i) Francis experiment : According to Francis electrophile first attacks on olefinic bond.
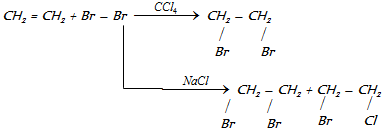
(ii) Reaction with hydrogen :

(iii) Reduction of alkene via hydroboration : Alkene can be converted into alkane by protolysis
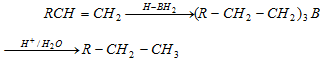
Hydroboration : Alkene give addition reaction with diborane which called hydroboration. In that reaction created trialkylborane, Which is very useful and used for synthesis of different organic compound
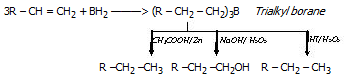
The overall result of the above relation seems to be antimarkownikoff's addition of water to a double bond.
(iv) By treatment with AgNO3 + NaOH : This reaction gives coupling
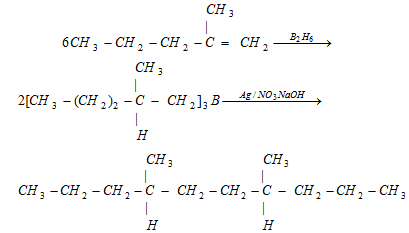
(v) Birch reduction : This reaction is believed to proceed via anionic free radical mechanism.
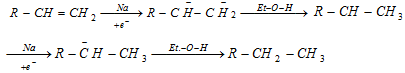
(vii) Reaction with HX [Hydrohalogenation]
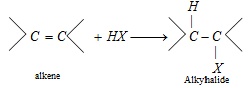
According to markownikoff's principle and kharasch effect.
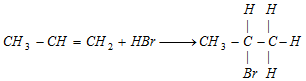
According to Anti Markownikoff principle
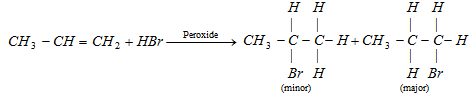
(viii) Reaction with hypohalous acids :
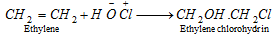
- In type of unsymmetrical alkenes markownikoff rule is followed.
(ix) Reaction with sulphuric acid :
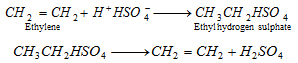
- This reaction is needed in the seperation of alkene from a gaseous mixture of alkanes and alkenes.
(x) Reaction with nitrosyl chloride
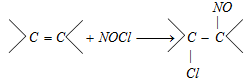 (NOCl is called Tillden reagent)
(NOCl is called Tillden reagent)
- If hydrogen is attached to the carbon atom of product, the product modifies to more stable oxime.
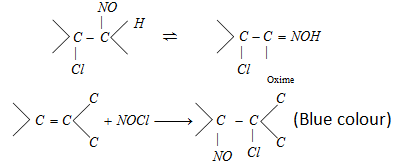
(xi) Ozonolysis
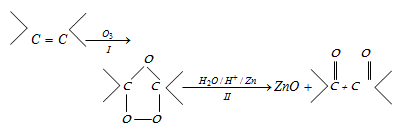
- Application of ozonolysis : This process is quite useful to locate the position of double bond in an alkene atom. The double bond is got by Joining the carbon molecules of the two carbonyl substances.
(xii) Oxy - mercuration demercuration : With mercuric acetate (in THF), followed by reduction with NaBH4/NaOH is also an example of hydration of alkene according to markownikoff's rule.
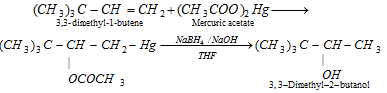
(xiii) Polymerisation
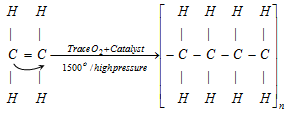
q If in polymerisation zeigler- natta catalyst [(R)3Al + TiCl4] is used then polymerisation is known as zeigler-natta polymerisation.
Uses
(i) For the manufacture of polythene - a plastic material; (ii) For artificial ripening of fruits; (iii) As a general anaesthetic; (iv) As a starting material for a large number of compounds such as glycol, ethyl halides, ethyl alcohol, ethylene oxide, etc; (v) For making poisonous mustard gas (War gas); (vi) For making ethylene-oxygen flame.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Chemical Properties of Alkenes questions? Chemical Properties of Alkenes topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Chemical Properties of Alkenes related problems. We provide step by step Chemical Properties of Alkenes question's answers with 100% plagiarism free content. We prepare quality content and notes for Chemical Properties of Alkenes topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours