Chemical properties of Alkanes
(i) Substitution reactions of Alkanes
(a) Halogenation : R-H + X-X → R-X +HX
The reactivity of halogen is : F2 > Cl2 > Br2 > I2
- Fluorine can react in dark Cl2, Br2 require light energy.I2 does not show any reaction at room temperature, but on heating it presents iodination.
- Iodination of methane is completed in presence of oxidising agent such as HNO3/HIO3/HgO which neutralizes HI.
- Chlorination of methane :
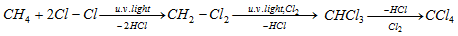
(ii) Reaction based on free radical mechanism
(a) Nitration : 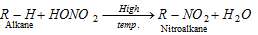
Nitrating mixture : (i) (Conc. HNO3 + Conc. H2SO4) at 2500 C
(ii) (HNO3 vapour at 400o - 500o C).
(b) Sulphonation : Free radical mechanism 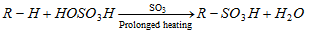
- Lower alkanes mainly ethane, methane do not give this reaction.
(iii) Oxidation
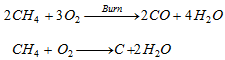
(a) Complete Oxidation or combustion :
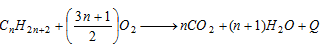
- This is exothermic reaction.
(b) Incomplete oxidation or combustion
(c) Catalytic Oxidation : 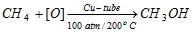
This is the industrial process for the developer of methyl alcohol.
- Higher alkanes are oxidised to fatty acids in presence of manganese stearate.
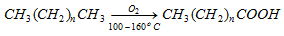
(d) Chemical oxidation :
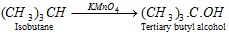
(iv) Thermal decomposition or cracking or pyrolysis or fragmentation
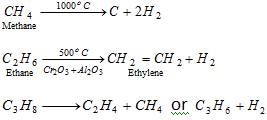
q This reaction is of great importance to petroleum industry.
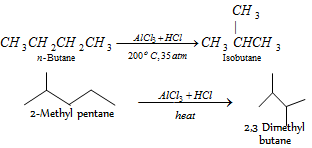
(v) Isomerisation :
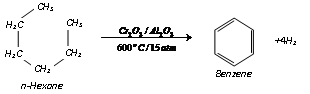
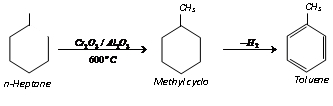
(vi) Aromatisation :
(vii) Step up reaction
(a) Reaction with CH2N2 (Diazo methane) :

(b) Reaction with CH3Cl/NaOH :
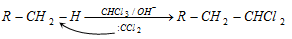
(c) Reaction with 
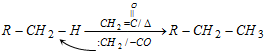
(viii) HCN formation :
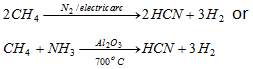
(ix) Chloro sulphonation/Reaction with SO2+Cl2
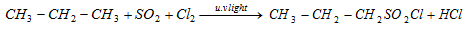
This reaction is called as reed's reaction.
q This is needed in the commercial formation of detergent.
(x) Action of steam : 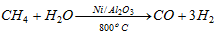
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Chemical properties of Alkanes questions? Chemical properties of Alkanes topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Chemical properties of Alkanes related problems. We provide step by step Chemical properties of Alkanes question's answers with 100% plagiarism free content. We prepare quality content and notes for Chemical properties of Alkanes topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours