Characteristics of catalysis
The below written are the characteristics which are common to most of the catalytic reactions.
(1) A catalyst remains unchanged in the mass and chemical composition at the end of the reaction.
(2) The small quantity of catalyst is generally sufficient to catalyses almost unlimited reactions
(i) For instance, in the decomposition of hydrogen peroxide, one gram of colloidal platinum can catalyses 108 litres of hydrogen peroxide.
(ii) In Friedel craft's reaction, the anhydrous aluminium chloride is needed in relatively large amount to the extent of 30% of the mass of benzene,
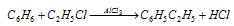
(3) The catalyst cannot initiate reaction: The function of the catalyst is to alter the speed of the reaction rather than to start it.
(4) The catalyst is commonly specific in nature: A substance, which acts as a catalyst for a particular reaction , fails to catalyse the other reaction , different catalysts for the same reactant may for different products.
Examples:
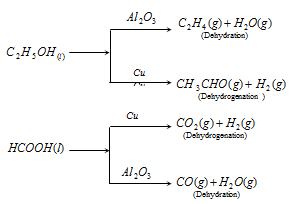
(5) Catalyst cannot change position of the equilibrium: The catalyst catalyze forward and backward reactions both to the same extent in a reversible reaction and thus have no effect on the equilibrium constant.
(6) Catalytic promoters: Substances which themselves are not catalysts, but when mixed in small quantities with the catalysts increase their efficiency are called as promoters or activators.
(i) For instance, in Haber's process for the synthesis of ammonia, traces of molybdenum increases the activity of finely divided iron which acts as a catalyst.
(ii) In the process of manufacture of methyl alcohol from water gas , chromic oxide is used as a promoter with the catalyst zinc oxide (ZnO).
(7) The Catalytic poisons: The substances which destroy the activity of the catalyst by their presence are known as catalytic poisons (i) For example, the presence of traces of arsenious oxide (As2O3) in the reacting gases reduces the activity of platinized asbestos which is used as catalyst in contact process for the manufacture of sulphuric acid.
(ii) The activity of the iron catalyst is destroyed by the presence of H2S or CO in the synthesis of ammonia by Haber's process.
(iii) The platinum catalyst taken in use in the oxidation of hydrogen is poisoned by CO.
(8) Change of temperature alters the rate of catalytic reaction as it does for the same reaction in absence of catalyst: By increasing the temperature, there is
Some increase in the catalytic power of a catalyst but after a definite temperature its power begins to decrease. A catalyst has therefore, a particular temperature at which the catalytic activity of it is maximum. This temperature is called as optimum temperature.
(9) The positive catalyst lowers activation energy
(i) In accordance to the collision theory, a reaction takes place on account of effective collisions between the reacting molecules.
(ii) For effective collision, it is necessary that the molecules must possess a minimum amount of energy known as activation energy (Ea).
(iii) After the collision molecules form the activated complex which dissociates to yield the product molecules.
(iv) The catalyst provides a new pathway including lower amount of activation energy. Thus,
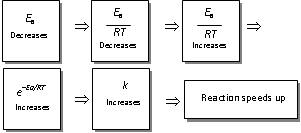
larger number of effective collisions occur in the presence of a catalyst in comparison to effective collisions at the same temperature in absence of a catalyst. Thus the presence of a catalyst makes the reaction to move faster.
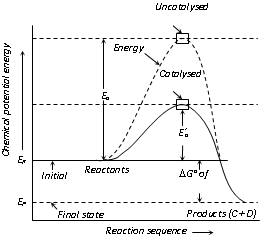
(v) Figure shows that the activation energy Ea, in the absence of a catalyst is higher than the activation energy Ea, in presence of a catalyst.
(vi) ER and EP represent the average energies of reactants and products. The difference gives the value of ΔG, that is ΔG=ER-EP
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Characteristics of catalysis questions? Characteristics of catalysis topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Characteristics of catalysis related problems. We provide step by step Characteristics of catalysis question's answers with 100% plagiarism free content. We prepare quality content and notes for Characteristics of catalysis topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours