General characteristics of arenes
(1) All arenes have general formula [CnH2n - 6y]. Where y is number of benzene rings and n is not less than 6.
(2) Arenes are planar and cyclic. They undergo replacement rather than addition reactions.
(3) Aromaticity or aromatic character : The characteristic behaviour of aromatic compounds is known as aromaticity. Aromaticity is because of extensive delocalisation of p-electrons in planar ring system. Huckel (1931) explained aromaticity on the basis of following rule.
Huckel rule : For aromaticity the atom can be planar, cyclic system having delocalised (4n + 2)Π electrons where n is an integer equal to 0, 1, 2, 3,------.
Thus, the aromatic molecules have delocalised electron cloud of 2,6,10 or 14 p electrons.
As like: 4n +2 = 6; 4n = 4; n = 4/4 = 1
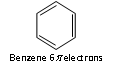 n = 1
n = 1
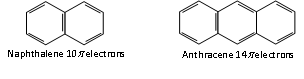
n= 2 n = 3
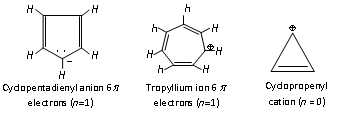
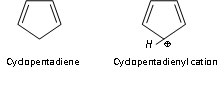
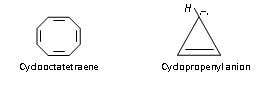
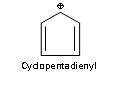
Similarly tropylium ion or cyclolpentadienyl anion are also aromatic because of containing 6p electrons (n=1).
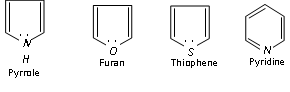
Hetrocyclic compounds also have 6p electrons (n = 1).
Atoms do not suit huckel principle are not aromatic.
4Π electrons 4Π electrons
8Π electrons 4Π electrons
(4) Antiaromaticity : Planar cyclic conjugated substances, less stable than the relating acyclic unsaturated species are called antiaromatic. Molecular orbital computation has given that such compounds have 4nΠ electrons. In fact these cyclic elements which have 4nΠ electrons are known as antiaromatic compounds and this characteristic is called antiaromaticity.
Example : 1,3-Cyclobutadiene, It is extremely unstable antiaromatic compound because it has 4nΠ electrons (n-1) and it is less constant than 1,3 butadiene by about 83.6 KJ mol-1.
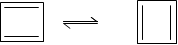 4n = 4 ; n = 4/4 = 1
4n = 4 ; n = 4/4 = 1
Thus, cyclobutanediene indicates two same contributing structures and it has n = 1.

4Π electrons 4Π electrons

8Π electrons 8Π electrons
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Characteristics of arenes questions? Characteristics of arenes topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors arXe available for 24x7 hours helping students in their Characteristics of arenes related problems. We provide step by step Characteristics of arenes question's answers with 100% plagiarism free content. We prepare quality content and notes for Characteristics of arenes topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours